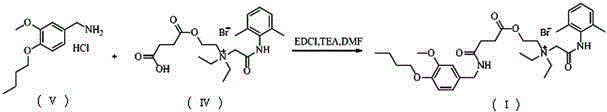
N-diethylaminoacetyl-2,6-dimethylaniline derivatives, preparation method and applications thereof
A technology of diethylaminoacetyl and dimethylaniline, applied in the field of N-diethylaminoacetyl-2,6-dimethylaniline derivatives, preparation and application, can solve the problem that QX314 cannot be used clinically, sensory nerve cells Blocking and other problems, to achieve ideal clinical application prospects and value, and good animal tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
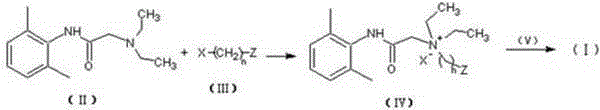
[0035] Preparation of intermediate compound (Ⅳ)
[0036]
[0037] Dissolve 5g of N-diethylaminoacetyl-2,6-dimethylaniline in 50ml of 2-bromoethanol, react in a closed container at 90°C for 24h, then slowly add the reaction solution dropwise into 200ml of anhydrous ether and After continuous stirring, a white solid was precipitated, filtered and dried to obtain 2.37 g of product (Ⅳ), with a yield of 31%.
[0038] 1 H NMR (400MHz, CD 3 OD) TM : 7.11~7.16 (m, 3H), 4.50~4.51 (m, 2H), 4.05~4.07 (m, 2H),3.75~3.87(m, 6H), 2.26 (s, 6H), 1.43(t, J = 7.2Hz, 6H).
[0039] 13 C NMR (400MHz, CD 3 OD) TM : 8.28, 18.65, 56.81, 56.93, 58.48, 61.63, 128.92, 129.31, 134.19,
[0040] 136.80, 164.15.
[0041] Detection instrument: Agilent 1200 series 6130 mass spectrometer
[0042] ESI: C 16 h 27 N 2 o 2 + , [M] + : 279.2075.
Embodiment 2
[0044] Preparation of intermediate compound (Ⅳ)
[0045] Dissolve 5g of N-diethylaminoacetyl-2,6-dimethylaniline in 50ml of 2-bromoethanol, react in a closed container at 50°C for 72h, then slowly add the reaction solution dropwise to 200ml of anhydrous ether and keep After stirring, a white solid was precipitated, filtered, and dried to obtain 2.06 g of product (IV), with a yield of 27%.
Embodiment 3
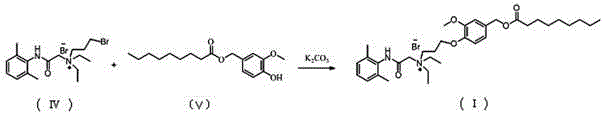
[0047]
[0048] Compound (IV) (250 mg, 0.70 mmol) was dissolved in 20ml of acetone, cooled to 0°C, and triphosgene (208 mg, 0.70 mmol) and pyridine (166 mg, 2.10 mmol) were added under nitrogen protection. After the addition, the cold bath was removed, the reaction solution was reacted overnight at room temperature, filtered, and the crude product of the acid chloride intermediate obtained after the filtrate was concentrated was directly fed.
[0049] Add 20ml of acetone to the acid chloride intermediate, add artificial capsaicin (Ⅴ) (308 mg, 1.05 mmol), pyridine (111 mg, 1.40 mmol), stir the liquid at room temperature under nitrogen protection, filter, concentrate, and obtain by preparative chromatography Yellow softened solid product (I) 45 mg, two-step yield 9.5%. Test results:
[0050] 1 H NMR (400MHz, CD 3 OD) TM : 7.10~7.15 (m, 3H), 7.04 (s, 1H), 6.94 (d, J = 8.0Hz, 1H), 6.83 (d, J = 8.0Hz, 1H), 4.76 (m, 2H), 4.48 ( s, 2H), 4.36 (s, 2H), 4.20 (m, 2H), 3.76~3.83(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com