Naphthalene tetracarboxyldiimide copolymer containing thienopyrroledione unit, and preparation method and application thereof
A unit naphthalene tetracarboxylic acid diimide copolymer and a unit naphthalene tetracarboxylic acid diimide technology are applied in the field of optoelectronic materials and can solve the problems of low conversion efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
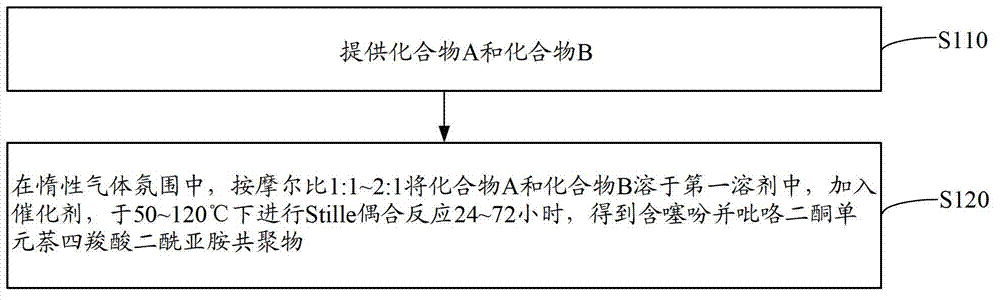
[0062] see figure 1 , the preparation method of the diketopyrrole unit naphthalene tetracarboxylic acid diimide copolymer containing thienopyrrole unit of an embodiment, comprises the following steps:
[0063] Step S110: providing compound A and compound B.
[0064] The structural formula of compound A is:
[0065]
[0066] Among them, R 1 for C 1 ~C 20 The alkyl group or the following structural unit:
[0067]
[0068] above R 2 , R 3 , R 4 for H, C 1 ~C 20 Alkyl or C 1 ~C 20 of alkoxy. R 2 , R 3 , R 4 Can be the same or different.
[0069] C above 1 ~C 20 The alkyl group is C 1 ~C 20 straight chain alkyl or C 1 ~C 20 branched chain alkyl. C 1 ~C 20 The alkoxy group is C 1 ~C 20 Linear alkoxy or C 1 ~C 20 branched chain alkoxy.
[0070] Compound A is prepared as follows:
[0071] Compound C and Compound D are provided:
[0072] Compound C has the following structure:
[0073]
[0074] Compound D is R 1 -NH 2 , where R 1 for C 1 ~C...
Embodiment 1
[0118] Poly(N,N'-di-octyl-1,4,5,8-naphthalene tetracarboxylic diimide-5-methyl-1,3-di(thiophen-2-yl)-4H-thiophene[ Preparation of 3,4-c]pyrrole-4,6(5H)-dione
[0119] (1) Preparation of N,N'-dioctyl-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide (Compound A)
[0120] The reaction formula is:
[0121]
[0122] Compound C and compound D are provided, compound C is 2,6-dibromo-1,4,5,8-naphthalene dianhydride, and compound D is n-octylamine (C 8 h 17 NH 2 );
[0123] Under nitrogen protection, n-octylamine (compound D) (0.13 g, 1 mmol) was added to propionic acid containing 2,6-dibromo-1,4,5,8-naphthalene dianhydride (0.43 g, 0.1 mmol) (15mL) solution, refluxed for 12 hours. After cooling to room temperature, the reaction solution was poured into aqueous sodium hydroxide solution and extracted with chloroform. The organic solvent was removed, washed with ethyl acetate, dissolved in chloroform, and then subjected to column chromatography with an alumina colu...
Embodiment 2
[0135] Poly(N,N'-di-((1-octylnonyl))-1,4,5,8-naphthalene tetracarboxylic acid diimide-5-(1-octylnonyl)-1,3-di Preparation of (thiophen-2-yl)-4H-thien[3,4-c]pyrrole-4,6(5H)-dione
[0136] (1) Preparation of N,N'-di-(1-octylnonyl)-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid imide (compound A)
[0137] The reaction formula is:
[0138]
[0139] Provide compound C and compound D, compound C is 2,6-dibromo-1,4,5,8-naphthalene dianhydride, compound D is 1-octyl-nonylamine;
[0140] Under nitrogen protection, 1-octyl-nonylamine (compound D) (0.255 g, 1 mmol) was added to 2,6-dibromo-1,4,5,8-naphthalene dianhydride (compound C) ( 0.43g, 0.1mmol) of propionic acid (15mL) solution, reflux for 14 hours. After cooling to room temperature, the reaction solution was poured into aqueous sodium hydroxide solution and extracted with chloroform. The organic solvent was removed, washed with ethyl acetate, dissolved in chloroform, and then subjected to column chromatography with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com