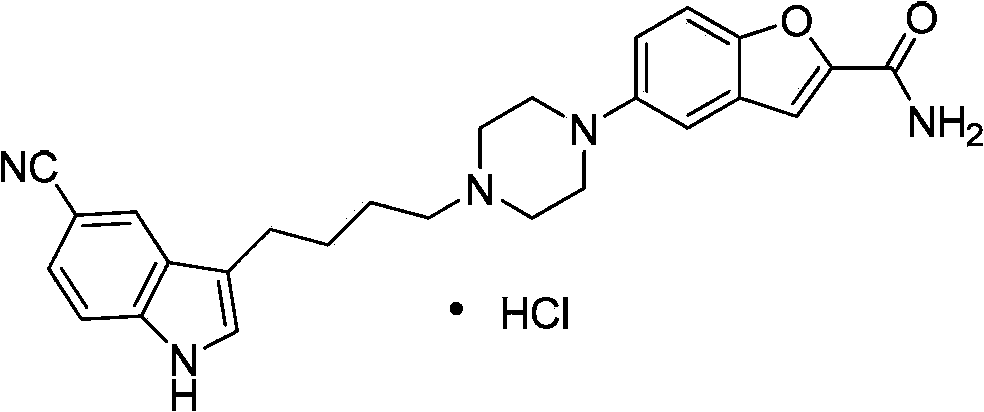
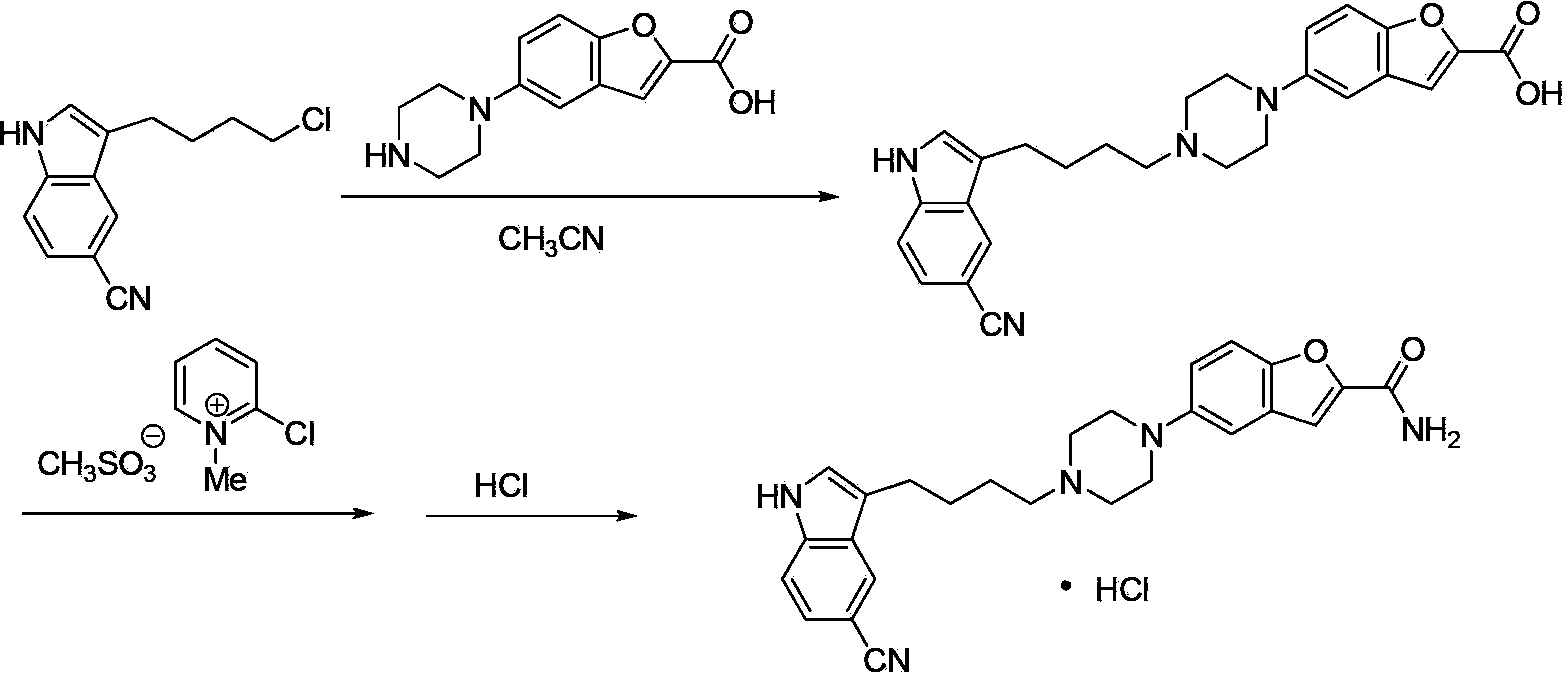
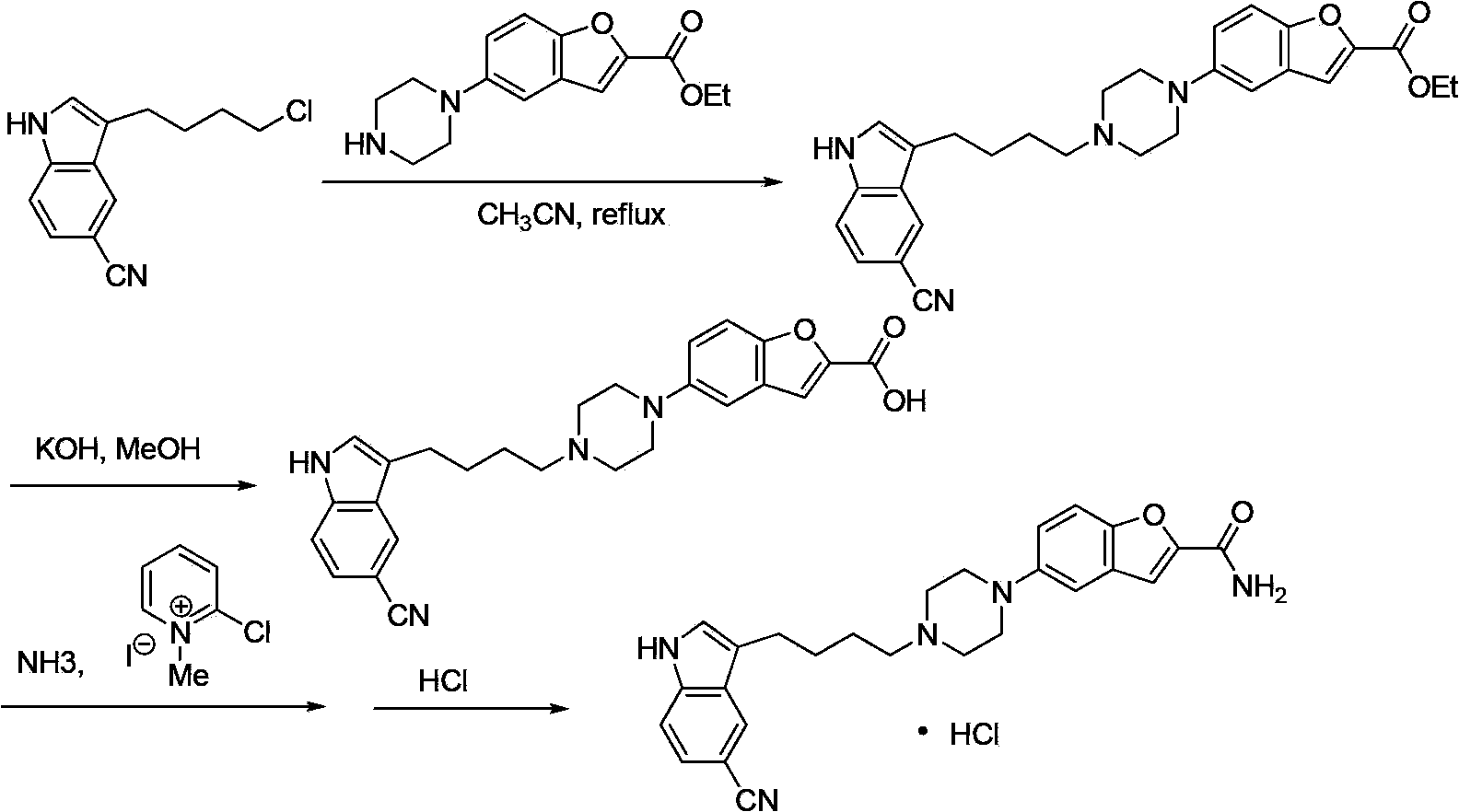
Compound for preparing vilazodone as well as intermediate and application thereof
A technology for compound and indole, applied in the field of compounds for preparing vilazodone, can solve problems such as being unsuitable for mass preparation of vilazodone hydrochloride, and achieve the effects of stable and controllable quality, suitability for industrial production, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of 3-(4-chlorobutyryl)indole-5-carbonitrile
[0066]
[0067] At 0°C, add 1,2-dichloroethane (400 mL) and anhydrous aluminum trichloride (40.0 g, 0.3 moL) into a 2 L three-necked flask equipped with mechanical stirring. At the same temperature, 4-chlorobutyryl chloride (42.2 g, 0.33 moL) was added dropwise, and the dropwise addition was completed in about half an hour, and the stirring was continued for 30 min. Then a solution of 5-cyanindole (36.4 g, 0.25 moL) in 1,2-dichloroethane (400 mL) was added, keeping the temperature at 0-5°C. The dropwise addition was completed within 30 minutes. Remove the ice bath, return to room temperature and continue to stir for 2h.
[0068] Next, 220 g of ice and 220 mL of concentrated hydrochloric acid were added to the reaction mixture. The reaction was stirred overnight at room temperature. After filtration and vacuum drying, 53.8 g of brown solid 3-(4-chlorobutyryl)indole-5-carbonitrile was obtained, with a yield ...
Embodiment 2
[0071] Preparation of 3-(4-bromobutyryl)indole-5-carbonitrile
[0072]
[0073] At 0°C, add 1,2-dichloroethane (200 mL) and anhydrous aluminum trichloride (15.4 g, 0.12 moL) into a 1 L three-necked flask equipped with mechanical stirring. At the same temperature, 4-bromobutyryl chloride (25.0 g, 0.13 moL) was added dropwise, and the addition was completed in about 20 minutes, and the stirring was continued for 30 minutes. Then a solution of 5-cyanindole (14.0 g, 0.096 moL) in 1,2-dichloroethane (200 mL) was added, keeping the temperature at 0-5°C. The dropwise addition was completed within 30 minutes. Remove the ice bath, return to room temperature and continue to stir for 2h.
[0074] Next, 100 g of ice and 100 mL of concentrated hydrochloric acid were added to the reaction mixture. The reaction was stirred overnight at room temperature. After filtration and vacuum drying, 21.8 g of brown solid 3-(4-bromobutyryl)indole-5-carbonitrile was obtained, with a yield of 78%. ...
Embodiment 3
[0077] Preparation of 3-(4-chlorobutyryl)-1-(naphthalene-1-ylsulfonyl)-1H-indole-5-carbonitrile
[0078]
[0079] Suspend NaH (3.0 g, 157.5 mmol) in dry DMF (150 mL), then cool to 0 °C, add 3-(4-chlorobutyryl)indole-5-carbonitrile (15.5 g, 63 mmol) in DMF (150 mL) solution. The mixture was stirred at room temperature for 30 min, then cooled to 0°C again, and 1-naphthalenesulfonyl chloride (15.7 g, 69 mmol) was added dropwise. The mixture was stirred overnight at room temperature.
[0080] Add saturated NH to the reaction mixture 4 Cl aqueous solution 100mL, then extracted with diethyl ether (150mL×3), the organic phase was washed with saturated brine, and dried over anhydrous magnesium sulfate. Filter, evaporate the solvent to dryness, and then recrystallize in acetone to obtain 20.8 g of white solid 3-(4-chlorobutyryl)-1-(naphthalene-1-ylsulfonyl)-1H-indole-5-carbonitrile , the yield was 76%.
[0081] ES Ⅰ-MS[m+1] + :437.
[0082] 1 H NMR (CDCl 3 , 500MHz, TMS) δ2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com