Preparation method of Ramelteon intermediate
A technology for ramelteon and intermediates, which is applied in the field of preparation of ramelteon intermediates, can solve the problems of unsuitability for large-scale industrial production, difficult control of reaction conditions, complicated reaction steps, etc., and achieve mature reaction mechanism and conditions Gentle, easy-to-step results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
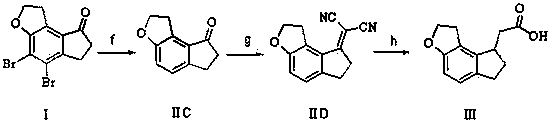
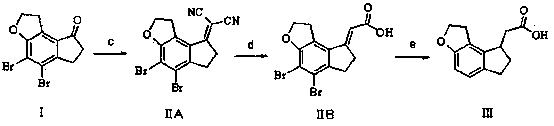
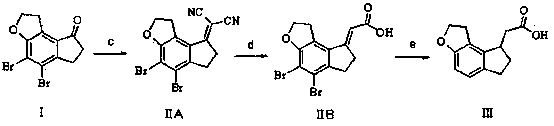
[0043] Example 1 (method 1)
[0044] At a temperature of 0-5°C, dissolve 3.4g of 60% NaH and 22.5g of 2-(diethoxyphosphono)acetic acid in 300mL of toluene, rise to room temperature and stir to dissolve, add 19.0g of compound I, Raise the temperature to 90-100°C and stir. The high performance liquid chromatography detects that the reaction is complete. The reaction liquid is cooled and added to water, stirred, the organic phase is separated, and dried to obtain 19.2 g of compound II with a yield of 89.7%.
[0045] 1.8 g of 10% Pd / C and 18.5 g of compound II were dissolved in 200 mL of ethanol, and hydrogen gas was introduced. TLC showed that the reaction was complete. After filtration, the filtrate was concentrated to obtain 9.58 g of intermediate III, with a yield of 88.9%.
[0046] Example 2 (method 1)
[0047]At a temperature of 0-5°C, dissolve 8.9g of potassium tert-butoxide and 21g of quaternary phosphonium triphenylacetate in 280mL of dimethyl sulfoxide, rise to room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com