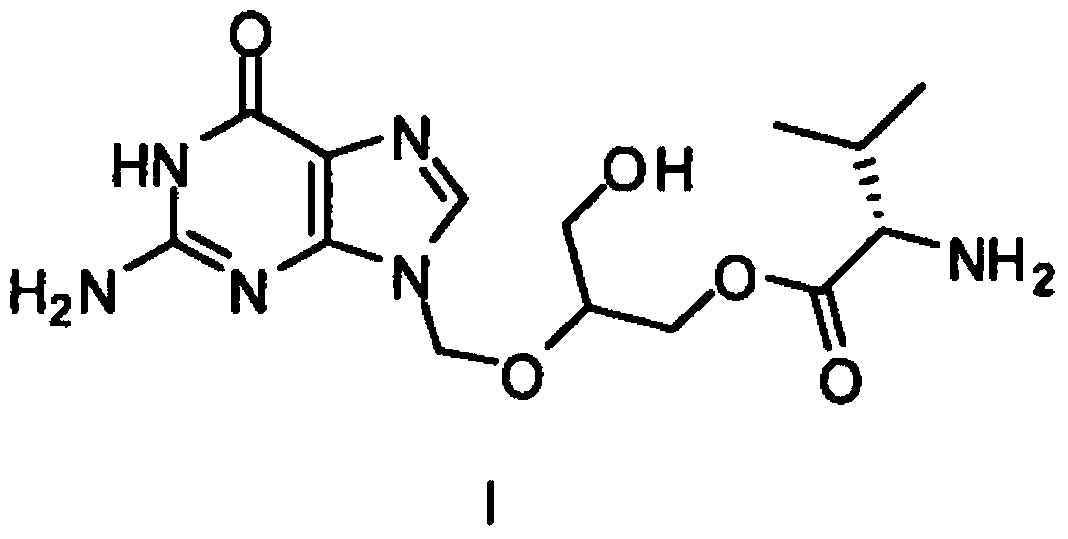
Process for the preparation of 2-amino-9-((2-phenyl-1,3-dioxan-5-yloxy)methyl)-1h-purin-6(9h)-one compound useful in the preparation of valganciclovir
The technology of a kind of compound, base oxygen, is used in the 2-amino-9-((2-phenyl-1,3-dioxan-5-yloxy)methyl group that is used for the preparation of valganciclovir )-1H-purin-6(9H)-one compound preparation field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
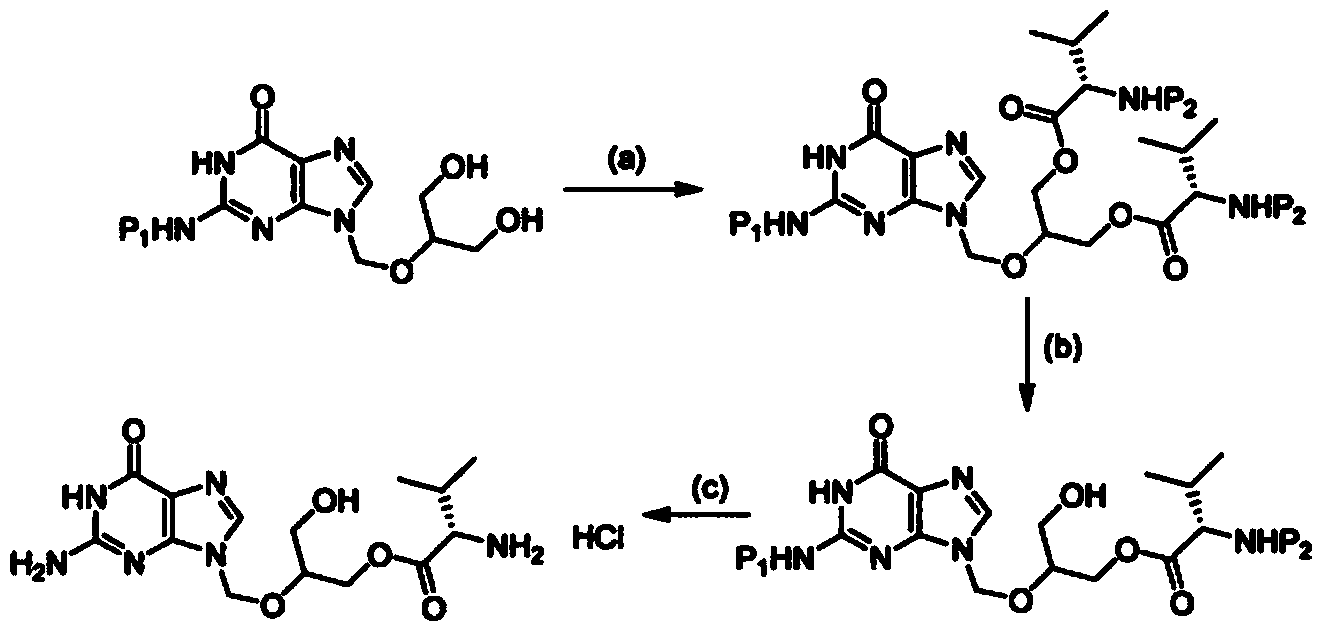
[0053] Example 1 : 2-amino-9-((2-phenyl-1,3-di Preparation of alk-5-yloxy)methyl)-1H-purin-6(9H)-one
[0054] Charge a 21t R.B. flask with 500ml DMSO and 100g 2-amino-9-((1,3-dihydroxypropan-2-yloxy)methyl)-1H-purine- 6(9H)-Kone (0.392 mol). The reaction mass is heated at a temperature of about 70° to about 80°C until all materials are dissolved. 129 ml of benzaldehyde dimethyl acetal (0.862 mol) and 2.6 g of p-toluenesulfonic acid monohydrate (0.014 mol) were slowly added to the above solution. The reaction mass was stirred at a temperature of about 70 to 80°C for about 12-18 hours. At the end of the reaction, 20 ml of triethylamine was added to the reaction mass and cooled to a temperature of about 25 to about 35°C. At this temperature, 100 ml of ethyl acetate was added with stirring and 1500 ml of D.M. water was added over 90-150 minutes, maintaining the temperature at 25-35°C (use external cooling if necessary). The reaction mass was stirred for an additional 300-3...
Embodiment 2
[0069] Example 2 : Preparation of 2-amino-9-((1-(benzyloxy)-3-hydroxyprop-2-yloxy)methyl)-1H-purin-6(9H)-one
[0070] 1500 ml THF and 1500 ml DCM were added to a 51t R.B. flask with stirring at a temperature of about 25° to about 30°C and cooled at a temperature of about 0°C to about 10°C. 272 g of aluminum chloride (1.893 mol) was added to the solvent mixture maintaining a temperature of about 0° to 10°C. Add sodium borohydride 77g (1.893mol), then add 100g 2-amino-9-((2-phenyl-l,3-di Alk-5-yloxy)methyl)-1H-purin-6(9H)-one (0.291 mol). Adjust the bubbler and add 6-8ml of D.M. water. The reaction mass was stirred at the same temperature for about 30 to 60 minutes, and was analyzed by TLC (CHCl 3 :MeOH60:10) Check progress. Add 4-6ml of D.M. water repeatedly until complete. The reaction mixture was quenched by dropwise addition of 500ml of D.M. water keeping the temperature within 2° to 8°C. An additional 500 ml of D.M. water was added with stirring and the reaction ma...
Embodiment 3
[0079] Example 3(a) : (2S)-2-((2-amino-6-oxo-1H-purin-9(6H)-yl)methoxy)-3-(benzyloxy)propyl-2-(benzyloxy Preparation of Carbonylamino)-3-Methylbutyrate
[0080] To a solution of 218.5 g (0.868 mol) of N-benzyloxycarbonyl-L-valine in 360 ml of DCM was added 71.7 g (0.347 mol) of dicyclohexylcarbodiimide under nitrogen. The mixture was stirred at room temperature for 20-30 hours. The resulting mixture was filtered to remove insoluble material, washed with 360 ml DCM and evaporated under reduced pressure. The foamy residue was dissolved in 100ml DMF and directly added to 100g 2-amino-9-((2-phenyl-1,3-di Alk-5-yloxy)methyl)-1H-purin-6(9H)-one (0.289 mol) in mixture. 4-Dimethylaminopyridine 8.83 g (0.072 mol) was added and the mixture was stirred under nitrogen for 18-24 hours. The reaction mass was poured into 6000 ml of D.M. water and extracted with 1750 ml of ethyl acetate and 1750 ml of toluene. The aqueous layer was separated and the organic layer was washed with 1000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com