Composite nickel hydroxide particles and nonaqueous electrolyte secondary battery
一种复合氢氧化物、非水电解质的技术,应用在非水电解质蓄电池、镍化合物、电池电极等方向,能够解决电池容量降低、选择性劣化等问题,达到粒度分布范围均质、粒度分布范围窄、抑制新核的形成的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
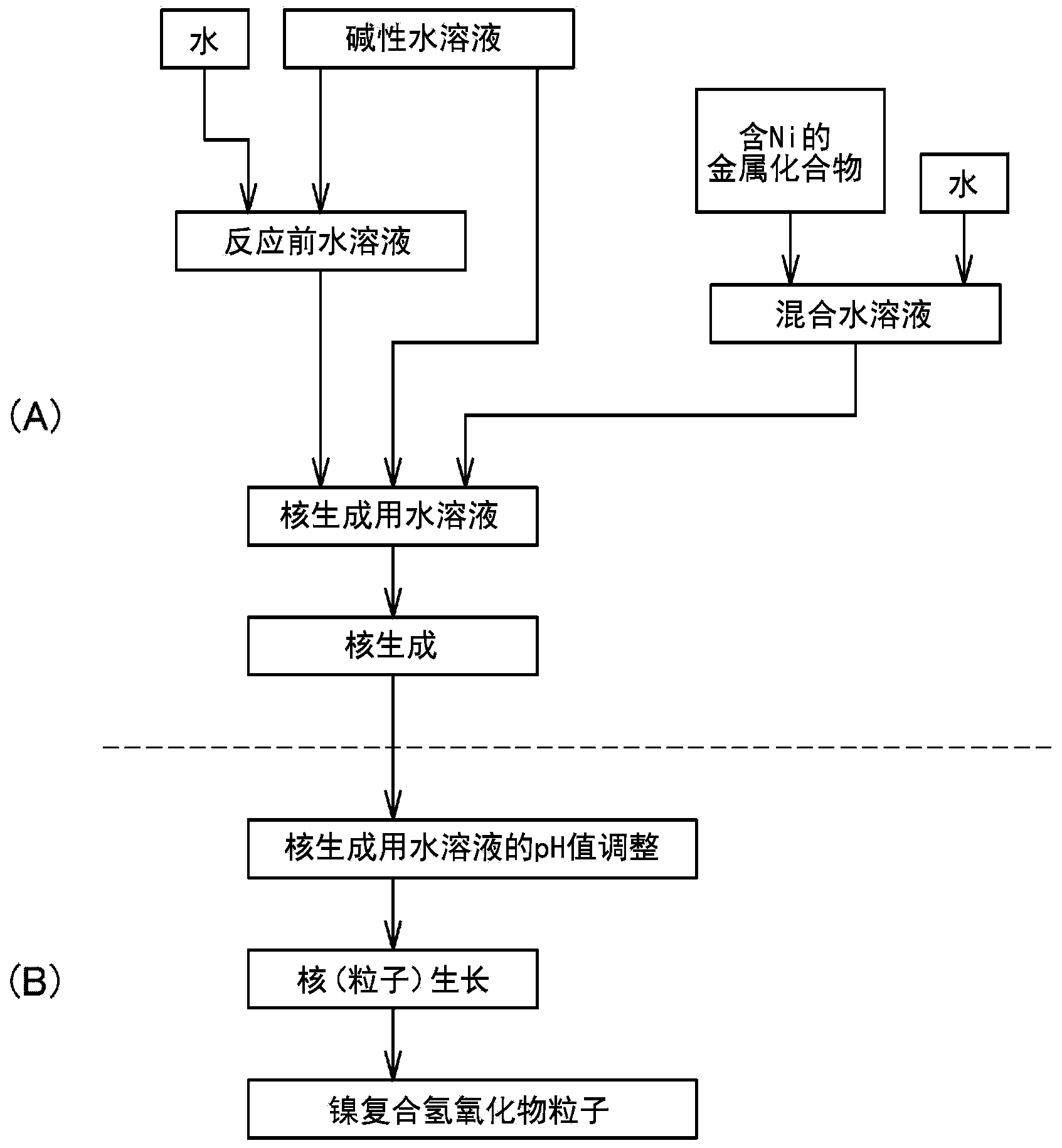
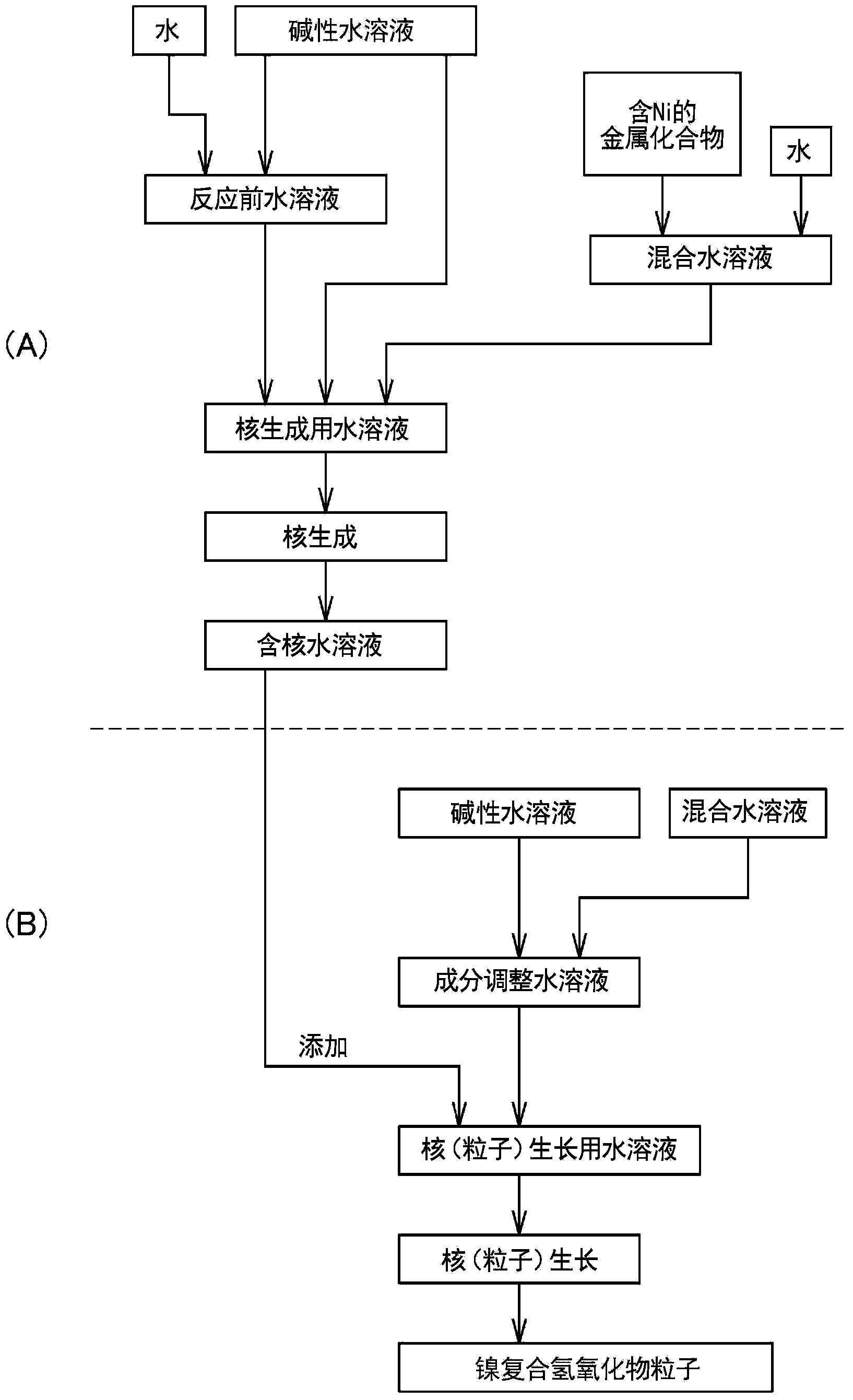
[0272] (B) Preparation of aqueous solution for nucleogenesis
[0273] An aqueous solution for nucleation can be prepared by mixing aqueous solutions of multiple metal compounds.
[0274] In addition, in the production method of the present invention, a solution that does not substantially contain a complex ion forming agent that forms a complex salt with a plurality of metal compounds is used as the aqueous solution for nucleation. The reason is that when the aqueous solution for nucleation contains a complex ion-forming agent, the solubility of nickel and cobalt increases, the precipitation rate of the composite hydroxide decreases, and the primary particles are easy to grow. The resulting nucleus, that is, the nucleus that cannot form the center of the secondary particle that is the composite hydroxide particle. An ammonium ion donor is mentioned as a typical complex ion forming agent. In addition, the above-mentioned "substantially does not contain" means that the content...
Embodiment 1)
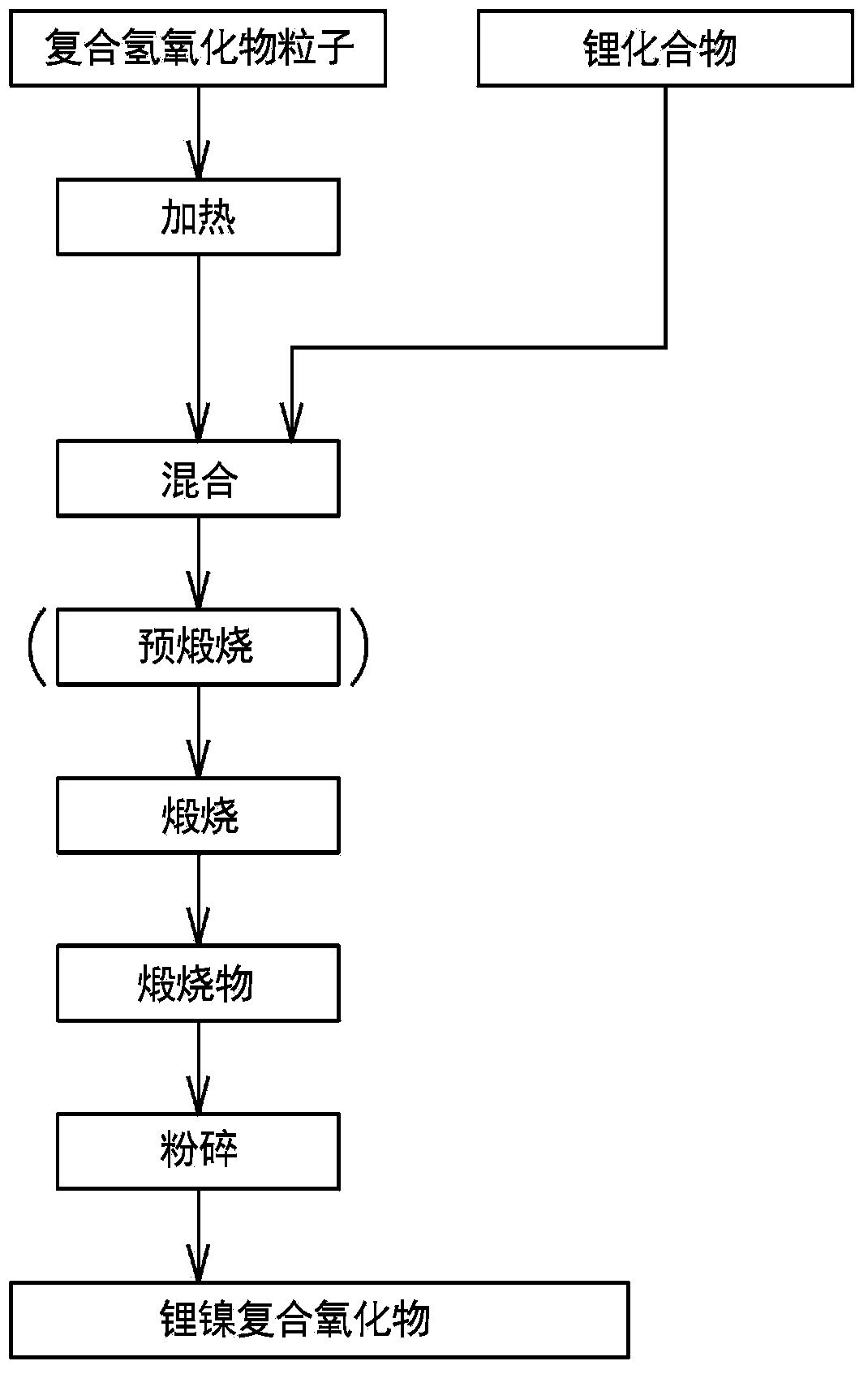
[0330] (Composite hydroxide manufacturing process)
[0331] Using the method of the present invention, composite hydroxides were produced as follows.
[0332] First, add water to the reaction tank (34L) to half the capacity of the reaction tank. While stirring, set the temperature in the tank to 70°C, and flow nitrogen into the reaction tank to form a nitrogen environment. At this time, the oxygen concentration in the space in the reaction tank was 2.0% by volume.
[0333] The pH value of the reaction solution in the tank was adjusted to 12.6 by adding an appropriate amount of 25% sodium hydroxide aqueous solution to the water in the aforementioned reaction tank, as a pH value at a liquid temperature of 25°C.
[0334] (nucleation process)
[0335] Next, a mixed aqueous solution of 1.8 mol / L was obtained by dissolving nickel sulfate and cobalt sulfate in water. In this mixed aqueous solution, the molar ratio of each metal element was adjusted so as to achieve Ni:Co=0.76:0.14...
Embodiment 2)
[0356] Except for washing with water after calcination, and mixing with water so that the slurry concentration becomes 1000g / L, filtering and drying after stirring, the same operation as in Example 1 was carried out to obtain a positive active electrode for a non-aqueous electrolyte secondary battery. substance. The performance of the obtained positive electrode active material for nonaqueous electrolyte secondary batteries was evaluated in the same manner as in Example 1.
[0357] In addition, by chemical analysis, it was confirmed that the composition of the obtained positive electrode active material was 7.27% by mass of Li, 46.7% by mass of Ni, 8.87% by mass of Co, 2.89% by mass of Al, and Li 0.994 Ni 0.76 co 0.14 al 0.10 o 2 , and is a hollow structure; by powder X-ray diffraction, it is confirmed that there is a single phase of lithium-nickel-cobalt composite oxide having a hexagonal layered crystal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com