Method for binding and fixing ionic type iridium coordination compound based on covalent bond
An iridium complex, ionic technology, applied in the field of solid-phase electrochemiluminescence electrodes, can solve the problems of easy contamination, poor reproducibility, short service life, etc., and achieve stable existence, good reproducibility, and good response. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
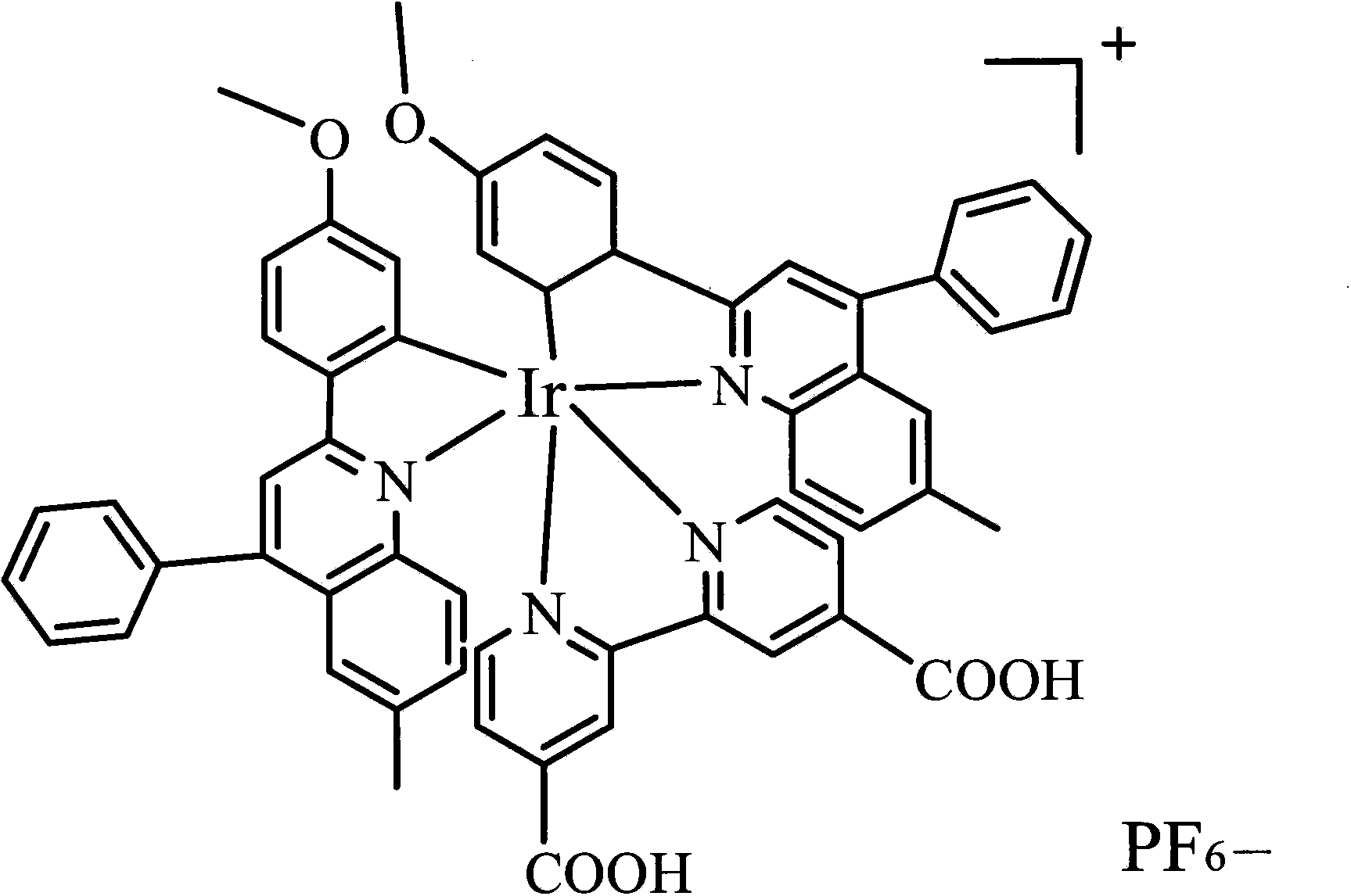
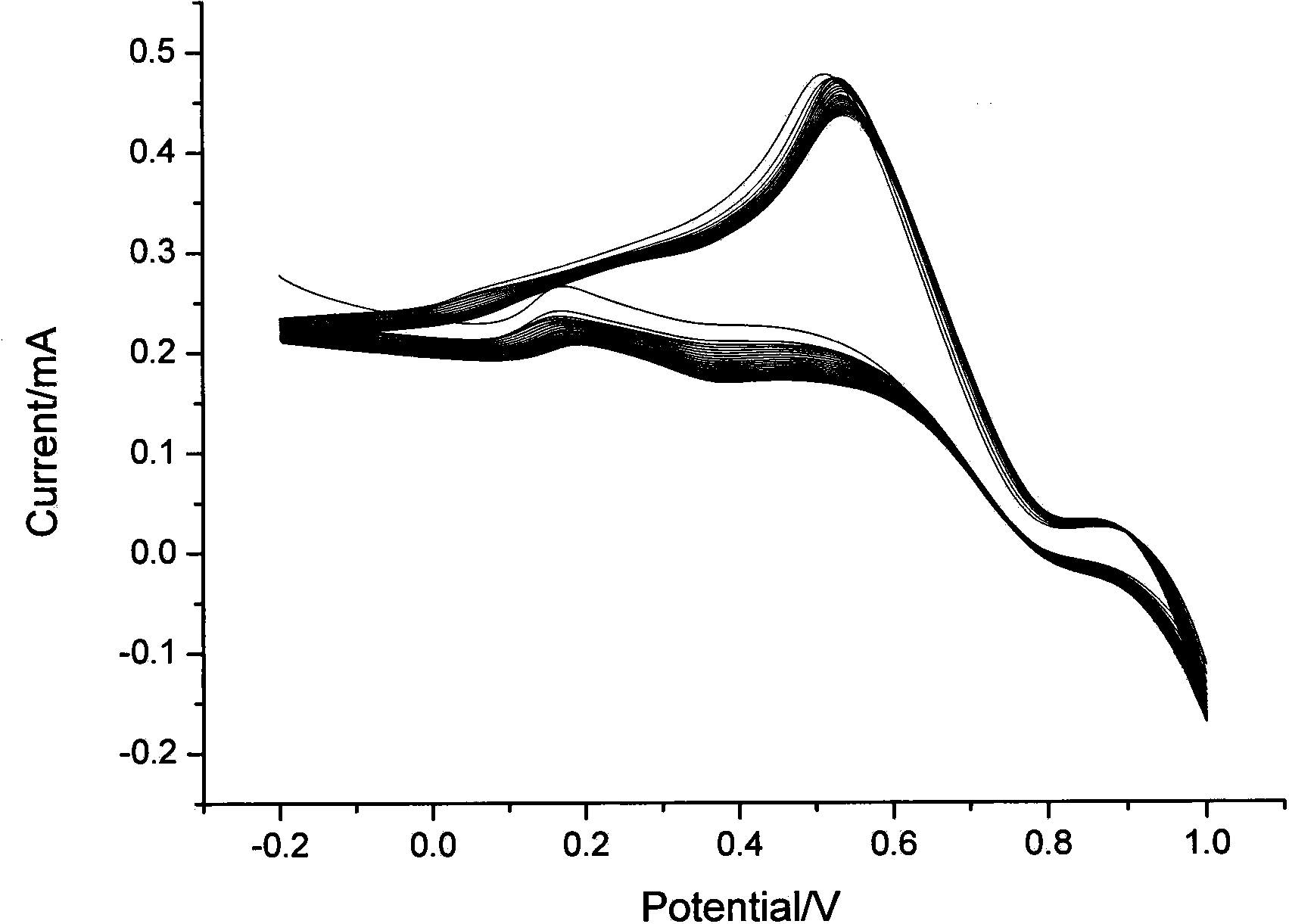
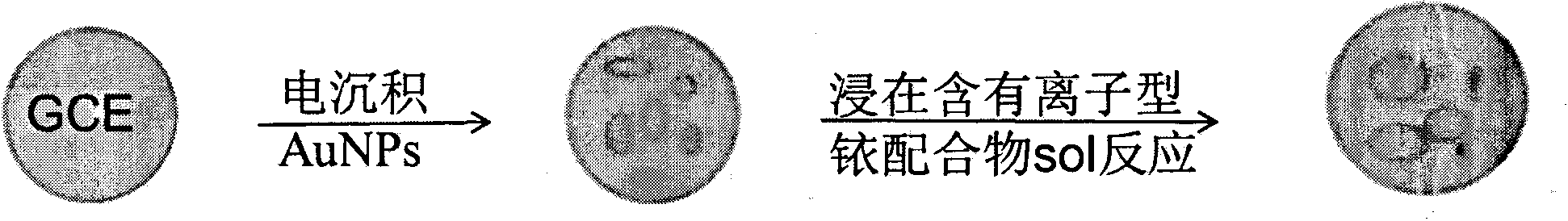
[0019] Example 1. Preparation of sensor: A certain amount of EDC and NHS were dissolved in acetonitrile, and the appropriate concentration ranges were: EDC 5%, NHS 2%. After dissolving, add ionic iridium complex (amount of 2%), stir evenly, and obtain (dpq-OCH 3 ) 2Ir(dcbpy)-NHS ester intermediate. Then, 0.02% of mercaptoethylamine was added into the solution, and stirred to carry out a sufficient reaction to obtain an iridium complex solution with mercapto groups. Take another glassy carbon electrode, after it has been polished, it is cleaned with water and ethanol in sequence, and then electrodeposition is used to fix nano-gold particles for immobilizing ionic metal iridium complexes. The method of electrodeposition is: insert the glassy carbon electrode into the 2 SO 4 and 3mmol / L HAuCl 4 The glassy carbon electrode loaded with gold nanoparticles was prepared by cyclic voltammetry. The scanning potential range is -0.2~1.0V, and the scanning speed range is 10mV / S. Fin...
example 2
[0020] Example 2. Preparation of sensor: A certain amount of EDC and NHS were dissolved in acetonitrile, and the appropriate concentration ranges were: EDC 5%, NHS 2%. After dissolving, add ionic iridium complex (0.1%), stir evenly, and obtain (dpq-OCH 3 ) 2 Ir(dcbpy)-NHS ester intermediate. Then, 0.05% of mercaptoethylamine was added into the solution, and stirred for a sufficient reaction to obtain an iridium complex solution with mercapto groups. Take another glassy carbon electrode, after it has been polished, it is cleaned with water and ethanol in sequence, and then electrodeposition is used to fix nano-gold particles for immobilizing ionic metal iridium complexes. The method of electrodeposition is: the glassy carbon electrode is inserted into a layer containing 1mol / L Na 2 SO 4 and 1mmol / L HAuCl 4 The glassy carbon electrode loaded with gold nanoparticles was prepared by cyclic voltammetry. The scanning potential range is -0.2~1.0V, and the scanning speed range i...
example 3
[0021] Example 3. Preparation of the sensor: A certain amount of EDC and NHS were dissolved in acetonitrile, and the appropriate concentration ranges were: EDC 10%, NHS 5%. After dissolving, add ionic iridium complex (amount of 1%), stir evenly, and obtain (dpq-OCH 3 ) 2 Ir(dcbpy)-NHS ester intermediate. Then, 0.05% of mercaptoethylamine was added into the solution, and stirred for a sufficient reaction to obtain an iridium complex solution with mercapto groups. Take another glassy carbon electrode, after it has been polished, it is cleaned with water and ethanol in sequence, and then electrodeposition is used to fix nano-gold particles for immobilizing ionic metal iridium complexes. The method of electrodeposition is: the glassy carbon electrode is inserted into a layer containing 2mol / L Na 2 SO 4 and 1mmol / L HAuCl 4 The glassy carbon electrode loaded with gold nanoparticles was prepared by cyclic voltammetry. The scanning potential range is -0.2~1.0V, and the scanning ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com