Tetrahydrothienopyridine deuterated derivatives, preparation method and pharmaceutical use thereof
A technology for tetrahydrothienopyridine and its derivatives, which is applied in the field of N--tetrahydrothienopyridine derivatives, drugs for the prevention or treatment of thrombosis and embolism-related diseases, and can solve the problem of inactive carboxylic acid derivatives that have not been metabolized , small dose, increased risk of bleeding, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
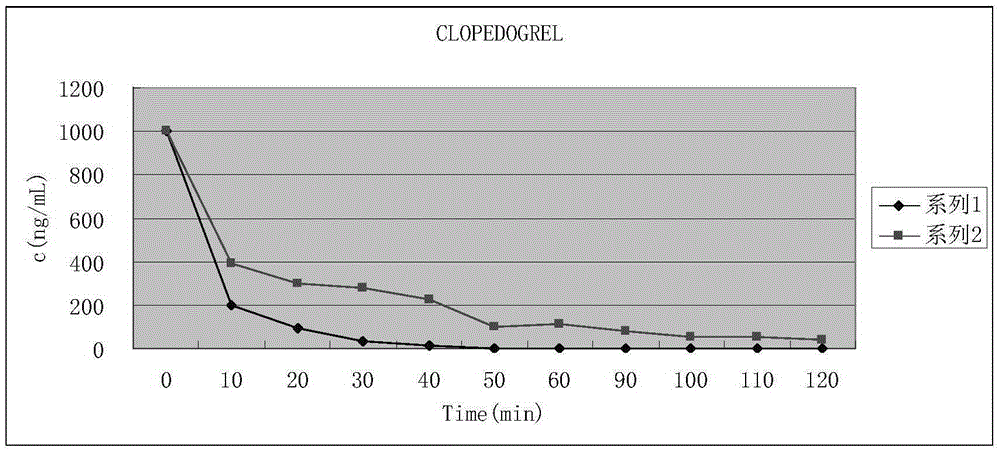
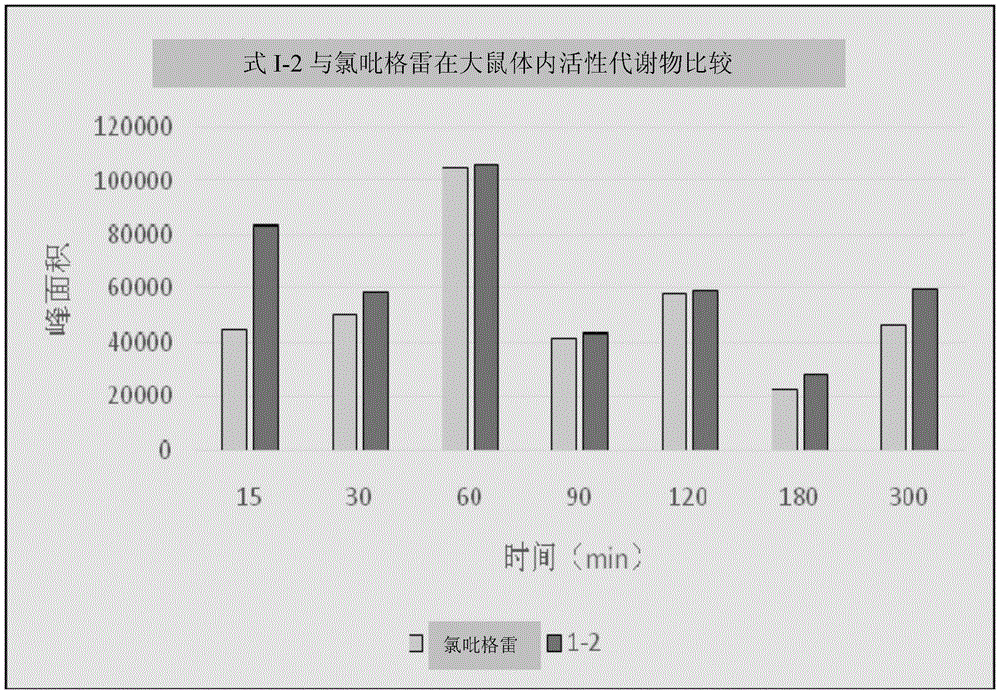
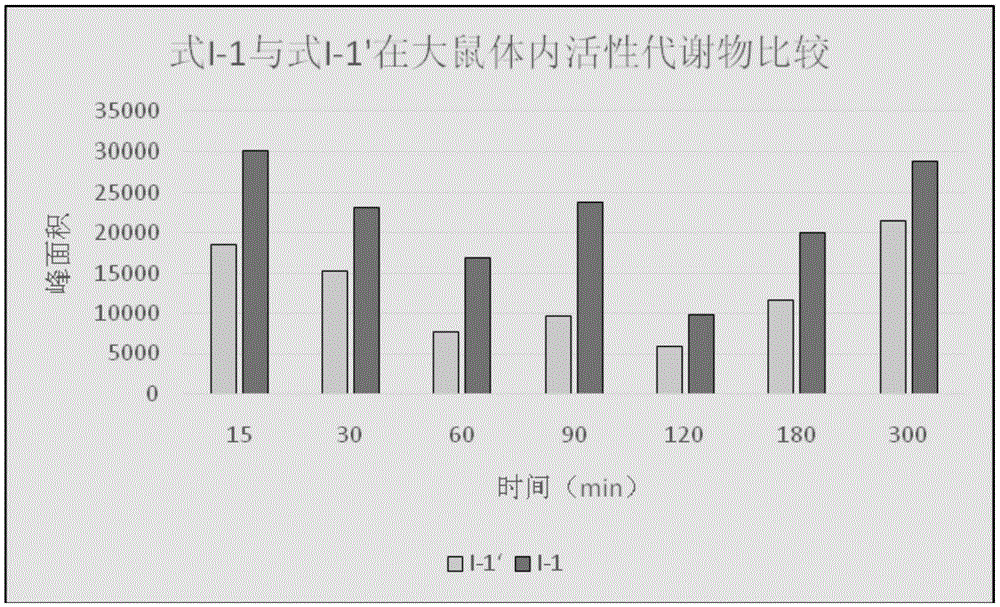
Image
Examples
Embodiment 1
[0084] (R)-Deuteromethyl o-chloromandelate
[0085]
[0086] Dissolve 9.4 g of (R)-o-chloromandelic acid in 36 mL of deuterated methanol, add 1 mL of 4M hydrogen chloride / dioxane solution, heat to reflux for 5 hours, evaporate the solvent under reduced pressure after cooling, and dissolve the residue in dichloromethane , washed with 5% potassium carbonate aqueous solution and water successively, dried the dichloromethane solution with anhydrous sodium sulfate, filtered to remove the desiccant and evaporated to dryness to obtain 9.2 g of colorless transparent oil (R)-deuterated methyl o-chloromandelate , yield 89.7%.
Embodiment 2
[0088] (R)-2-(2-Chlorophenyl)-2-(4-nitrobenzenesulfonyloxy)-deuteromethyl acetate (Ⅱ-1)
[0089]
[0090] Dissolve 10.2 g of (R)-deuteromethyl o-chloromandelate in 50 mL of anhydrous dichloromethane, add 65.6 g of triethylamine and a catalytic amount of DMAP, stir, cool down to 0°C, and add 12.2 g dropwise at the same temperature 50mL of anhydrous dichloromethane solution of p-nitrobenzenesulfonyl chloride, and then incubated for 4 hours. Add 100mL of water to the reaction solution, stir, let it stand, and separate the liquids. The water phase is then extracted three times with 150mL of dichloromethane. After combining the organic phases, dry them with anhydrous sodium sulfate. After filtering to remove the desiccant, evaporate the dichloromethane to dryness under reduced pressure. 20.9 g of a dark red oily crude product was obtained, and 15.8 g of a solid product (II-1) was obtained by methanol recrystallization, with a yield of 81.3%.
Embodiment 3
[0092] (2S)-2-(2-Chlorophenyl)-2-(2-oxo-7,7a-dihydrothieno[3,2-c]pyridin-5(2H,4H,6H)-yl) - deuteromethyl acetate (V-1)
[0093]
[0094] (R)-2-(2-chlorophenyl)-2-(4-nitrobenzenesulfonyloxy)-acetic acid deuterated methyl ester (II-1) 58.1g (0.15mol), 5,6, 7,7a-Tetrahydrothieno[3.2-c]pyridin-2(4H)-one hydrochloride (Ⅳ-1) 32.3g (0.17mol) and 37.8g (0.38mol) potassium bicarbonate were added in 500ml of acetonitrile , the reaction system was protected with nitrogen, and stirred at room temperature for 26 hours. After the reaction solution was allowed to stand, the insoluble matter was filtered to obtain a dark red mother liquor. The solvent was evaporated to dryness under reduced pressure, and 35.4 g of an oily product was obtained by flash column chromatography (petroleum ether: ethyl acetate = 4:1), with a yield of 70%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com