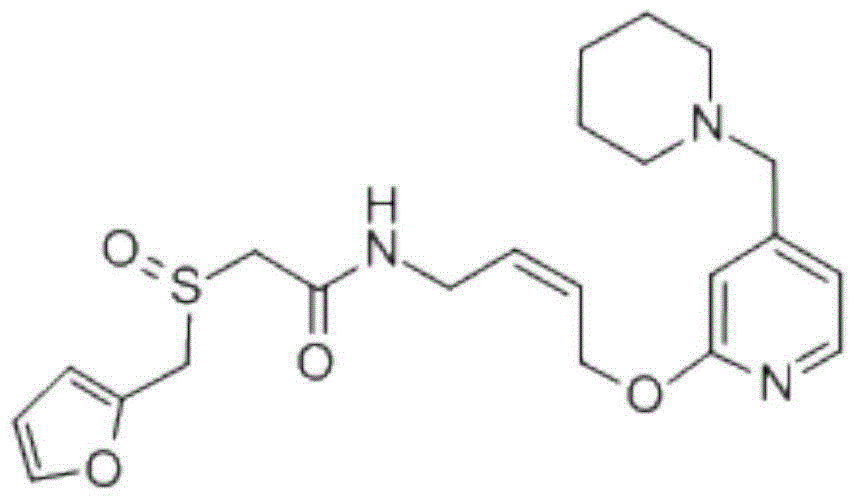
Lafutidine composition freeze-dried powder injection for injection
A technology of lafutidine and freeze-dried powder injection is applied in the field of medicine and medicine manufacturing, which can solve the problems of atrioventricular conduction block, no chitosan-containing nanoparticles, and pancytopenia, and achieves inhibition of the growth of harmful bacteria, The effect of promoting the reproduction of beneficial bacteria and eliminating the active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of lafutidine composition freeze-dried powder for injection, in 1000 pieces.
[0031] prescription:
[0032] Lafutidine 300g
[0033] Chitosan Nanoparticles 400g
[0034] Water for injection 2000ml
[0035] 2. Preparation process:
[0036] 1) Weigh 300g of lafutidine and slowly add it to 1800ml of water for injection, and stir until dissolved while adding.
[0037] 2) Weigh 400g of chitosan nanoparticles into the feed solution to dissolve and mix evenly.
[0038] 3) Add water for injection to the full amount, and detect the content of intermediates.
[0039] 4) Add 0.1% activated carbon, stir and adsorb for 30 minutes, filter out the activated carbon, and then filter the drug solution through 0.45 μm and 0.22 μm microporous membranes until clarification, and calculate the filling volume based on 0.3 g per bottle of lafutidine.
[0040] 5) Make the drug into a sterile aqueous solution, first cool down to -40±2°C during the freeze-drying process, stop co...
Embodiment 2
[0042] Preparation of lafutidine composition freeze-dried powder for injection, in 1000 pieces.
[0043] 1. Prescription:
[0044] Lafutidine 300g
[0045] Chitosan Nanoparticles 500g
[0046] Water for injection 2000ml
[0047] 2. Preparation process:
[0048] 1) Add the prescribed amount of lafutidine into 1800ml water for injection, and stir until dissolved while adding.
[0049] 2) Weigh 500g of chitosan nanoparticles into the feed solution to dissolve and mix evenly.
[0050] 3) Add water for injection to the full amount, and detect the content of intermediates.
[0051] 4) Add 0.1% activated carbon, stir and adsorb for 30 minutes, filter out the activated carbon, and then filter the drug solution through 0.45 μm and 0.22 μm microporous membranes until clarification, and calculate the filling volume based on 0.3 g per bottle of lafutidine.
[0052] 5) Make the drug into a sterile aqueous solution, first cool down to -40±2°C during the freeze-drying process, stop coo...
Embodiment 3
[0054] Preparation of lafutidine composition freeze-dried powder for injection, in 1000 pieces.
[0055] prescription:
[0056] Lafutidine 300g
[0057] Chitosan Nanoparticles 600g
[0058] Water for injection 2000ml
[0059] 2. Preparation process:
[0060] 1) Add the prescribed amount of lafutidine into 1800ml water for injection, and stir until dissolved while adding.
[0061] 2) Weigh 600g of chitosan nanoparticles into the feed solution to dissolve and mix evenly.
[0062] 3) Add water for injection to the full amount, and detect the content of intermediates.
[0063] 4) Add 0.1% activated carbon, stir and adsorb for 30 minutes, filter out the activated carbon, and then filter the drug solution through 0.45 μm and 0.22 μm microporous membranes until clarification, and calculate the filling volume based on 0.3 g per bottle of lafutidine.
[0064] 5) Make the drug into a sterile aqueous solution, first cool down to -40±2°C during the freeze-drying process, stop cooling,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com