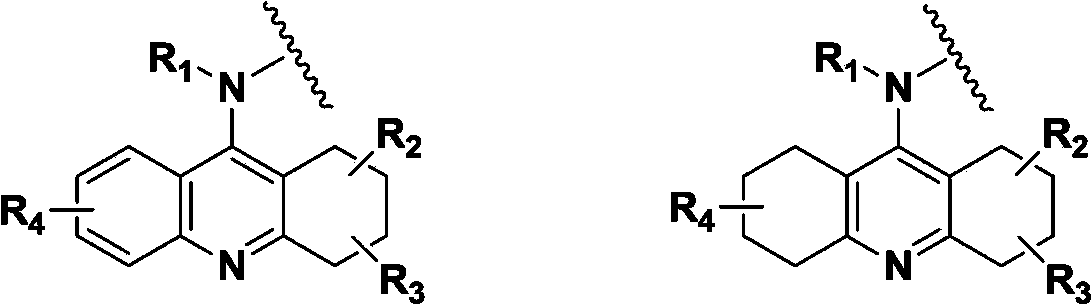
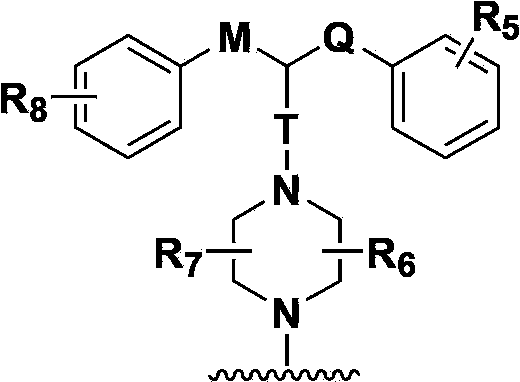
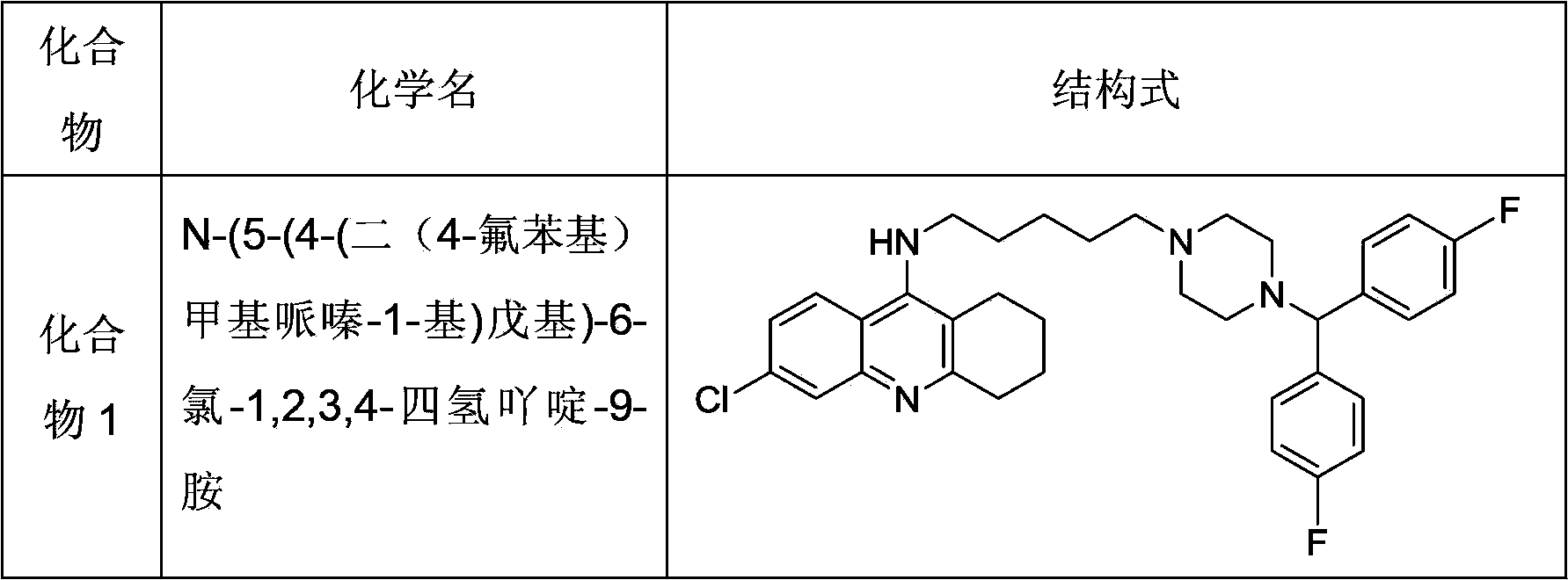
Hydrogenated acridine derivative and application thereof
A compound and chemical bond technology, applied in the application of calcium channel blockers or/and acetylcholinesterase inhibitors, in the field of compounds, can solve problems such as failure of nerve signal transmission, lack of acetylcholine, and effects on body cognition and memory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 prepares compound 1 provided by the invention
[0050] The preparation process is as follows:
[0051]
[0052] Accurately weigh 17.1g (0.1mol) of 4-chloro-2-aminobenzoic acid and 9.8g (0.1mol) of cyclohexanone, put them in an ice bath, slowly add 90mL of phosphorus oxychloride solution, and then slowly transfer the reaction system into the oil In the bath, slowly heat to reflux, stop the reaction after 2h, evaporate the phosphorus oxychloride solvent, dilute the remaining reactant with ethyl acetate, then slowly add potassium carbonate solution dropwise, adjust the pH value of the reaction solution to be greater than 8, separate the organic layer, wash with water , dried over anhydrous sodium sulfate, filtered and concentrated to obtain a dark viscous substance, to which an appropriate amount of acetone was added, heated to dissolve, placed in a refrigerator to crystallize, and filtered to obtain compound S111.0g, yield 43.8%, ESI-MS [M+H]+=252.0.
[...
Embodiment 2
[0059] Embodiment 2 prepares compound 2 provided by the invention
[0060] The preparation process is as follows:
[0061]
[0062] Accurately weigh 8.2g (60mmol) of anthranilic acid and 7.2g (73mmol) of cyclohexanone, put them in an ice bath, slowly add 50mL of phosphorus oxychloride solution, then slowly move the reaction system into an oil bath, and slowly heat to reflux , stop the reaction after 3h, evaporate the phosphorus oxychloride solvent, dilute the remaining reactant with ethyl acetate, then slowly add 2M NaOH solution dropwise, adjust the pH value of the reaction solution to be greater than 8, separate the organic layer, and extract the aqueous layer with ethyl acetate 3 times, the organic layer was combined, the organic layer was washed with saturated NaHCO3, dried over anhydrous sodium sulfate, filtered and concentrated, and the resulting residue was separated and purified by silica gel column chromatography to obtain the product S511.7g, the yield was 90%, ES...
Embodiment 3
[0070] Embodiment 3 prepares compound 3 provided by the invention
[0071] The preparation process is as follows:
[0072]
[0073]A mixed solution of 434mg about 2.0mmol of 9-chloro-1,2,3,4-tetrahydroacridine S8 and about 266mg of 2.2mmol of 2-(2-aminoethylmercapto)ethanol and 2ml of n-pentanol was heated to React under reflux at 145°C for 20h, cool to room temperature, add ethyl acetate to disperse, filter to obtain a dark brown solid, and dry to obtain crude product S9582mg, yield 96.4% ESI-MS [M+H]+=303.1.
[0074] Dissolve 582 mg of about 1.9 mmol of the above-prepared S9 in 8 mL of anhydrous dichloromethane, add 1.0 g of about 3.8 mmol of PPh3, and 1.26 g of about 3.8 mmol of CBr4 in the reaction system, stir at room temperature for 10 h, and detect by TLC After the reaction is complete, stop the reaction, add water to quench the reaction, extract the aqueous phase with CH2Cl2, wash the combined organic phase with saturated brine, dry over anhydrous sodium sulfate, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com