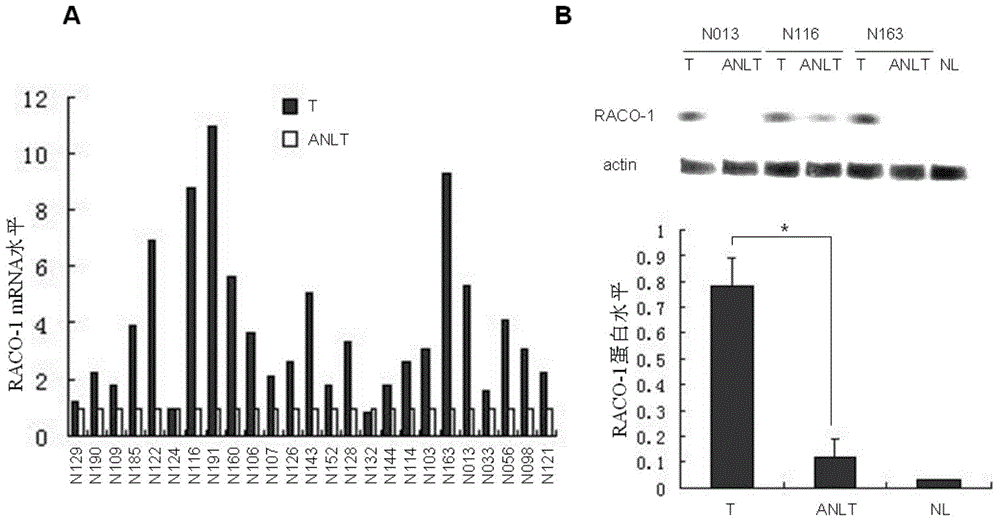
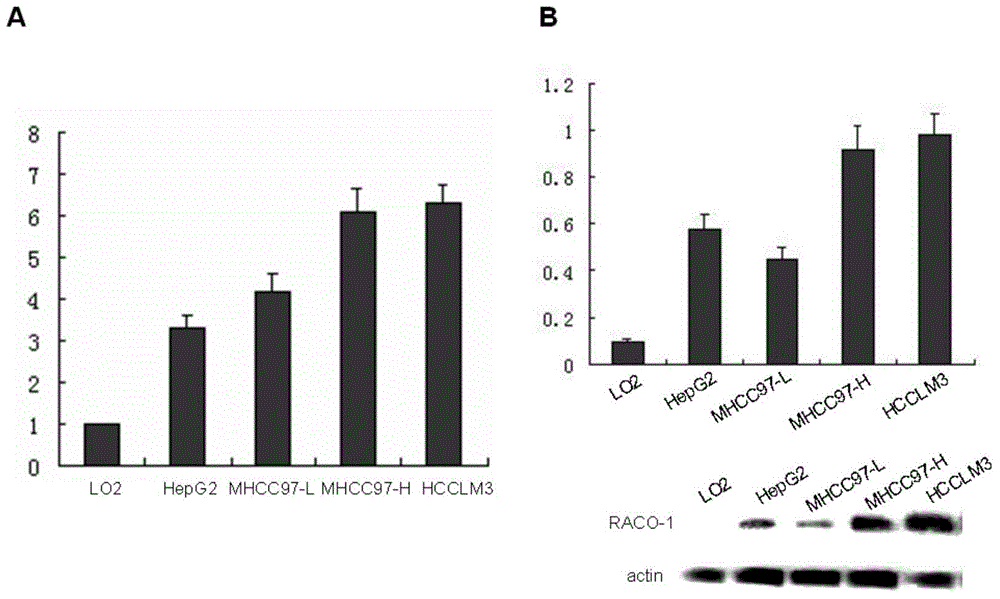
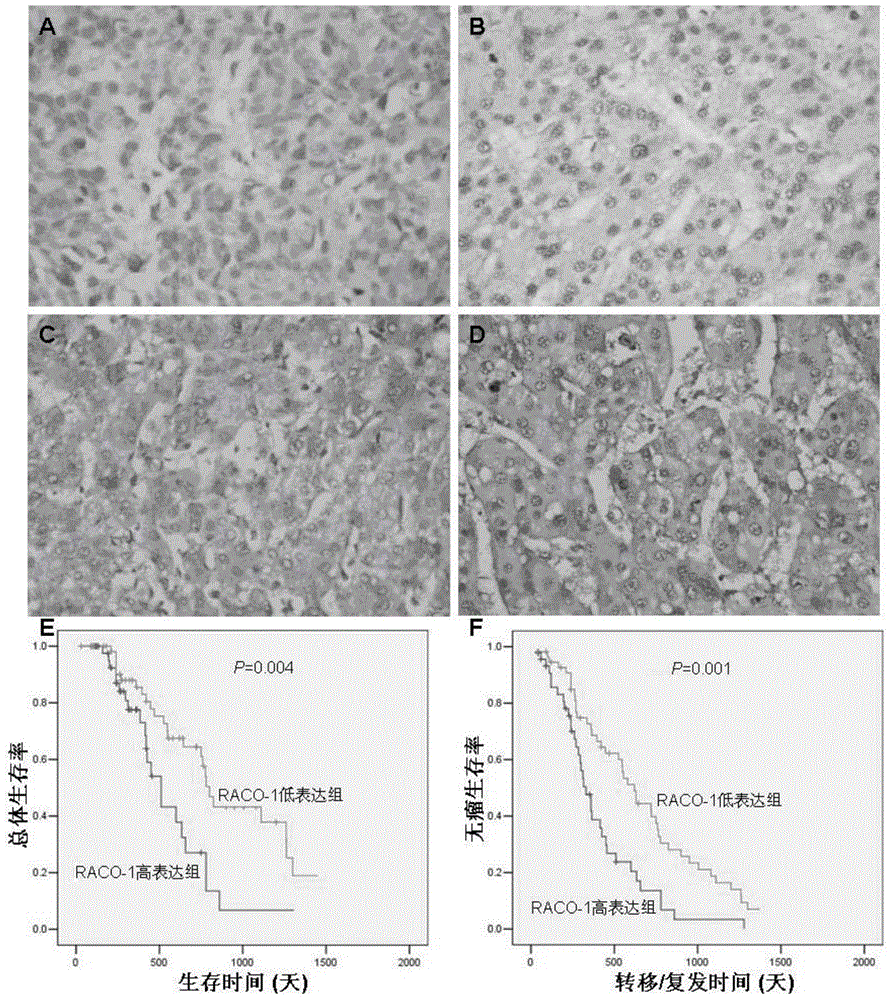
Drugs for treating liver cancer and application of raco-1 monoclonal antibody
A RACO-1 and monoclonal antibody technology, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., to achieve the effect of reducing the invasion and metastasis of liver cancer, improving the five-year survival rate, and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0040] According to another typical embodiment of the present invention, the sequence of the upstream primer used for cloning RACO-1 DNA is shown in SEQ ID NO: 3 (5'-TCAGGGAGAGAAAGGAAGACTG-3'); the sequence of the downstream primer is shown in SEQ ID NO: 4 (5 '-GTGGACAGAGCGACTTGGATAA -3').
[0041] In the present invention, an application of a RACO-1 monoclonal antibody in the preparation of a drug for treating liver cancer is provided, wherein the RACO-1 monoclonal antibody is a human RACO-1 monoclonal antibody, which interferes with the expression of the RACO-1 gene as a treatment for liver cancer. a method of
[0042] The present invention will be further described below in conjunction with embodiment.
Embodiment 1
[0044] 1) Extraction of total RNA and cloning of RACO-1 gene by reverse transcription PCR.
[0045] Total RNA in peripheral blood lymphocytes of patients with primary hepatocellular carcinoma with high expression of RACO-1 (primary hepatocellular carcinoma patient, female, 41 years old) was extracted by one-step method of guanidinium thiocyanate-phenol-chloroform. Take about 20 μg of total RNA, add 1 μg OligodT, 5ul 250mmol / LdNTP and 200UM-MLV reverse transcriptase (GIBCOLBRL), and carry out cDNA synthesis according to the product instructions. PCR amplification of the RACO-1 gene, wherein the amplification primers are as follows: the sequence of the upstream primer is SEQ ID NO: 1 NO: 1 (5'-aacaaggggtctgtggaaatc-3'); the sequence of the downstream primer is SEQ ID NO: 2 (5' -tgtagtcggtcagtgccttct-3'). The conditions of PCR amplification were: denaturation at 94°C for 20 min, annealing at 52°C for 50 s, extension at 72°C for 1.5 min, 35 cycles in total, and finally extension ...
Embodiment 2
[0059] PCR amplification of RACO-1 gene (from a patient with primary hepatocellular carcinoma, female, 48 years old), wherein the amplification primers are as follows: the sequence of the upstream primer is as shown in SEQ ID NO: 3 (5'- TCAGGGAGAGAAAGGAAGACTG-3' ); the sequence of the downstream primer is SEQ ID NO: 4 (5'- GTGGACAGAGCGACTTGGATAA -3'). All the other experimental conditions were the same as in Example 1.
[0060] In the following experiments, the RACO-1 monoclonal antibody was added to the cell culture medium to treat the cells.
[0061] The monoclonal antibody that embodiment 1 obtains is carried out in vitro test operation as follows:
[0062] The cells required for the experiment (HCCLM3 control and HCCLM3 anti-RACO-1 and MHCC97-H control and MHCC97-H anti-RACO-1 ), all instruments that can be sterilized are sterilized, and the ruler and marker pen are irradiated with ultraviolet rays for 30 minutes before operation. Digest the cells with 0.25% trypsin,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com