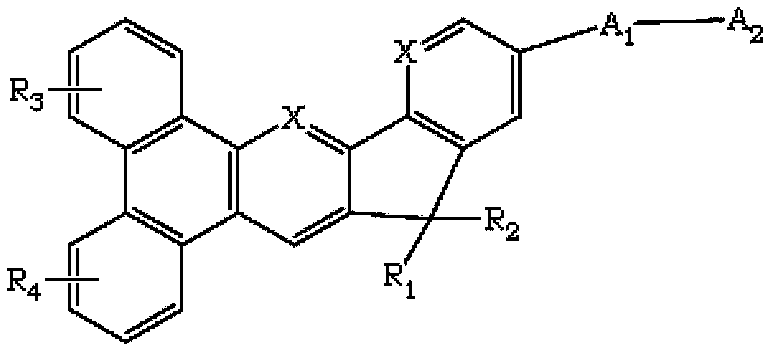
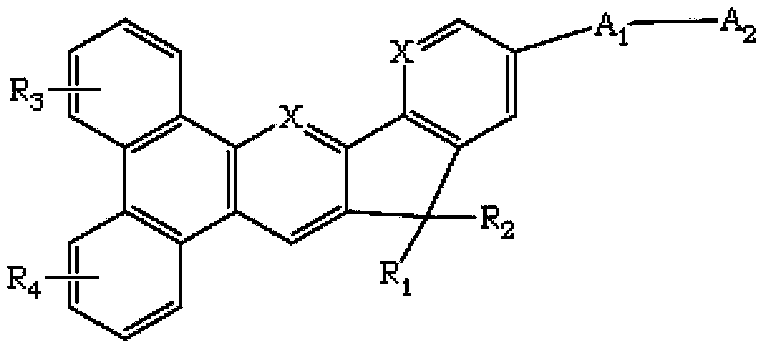
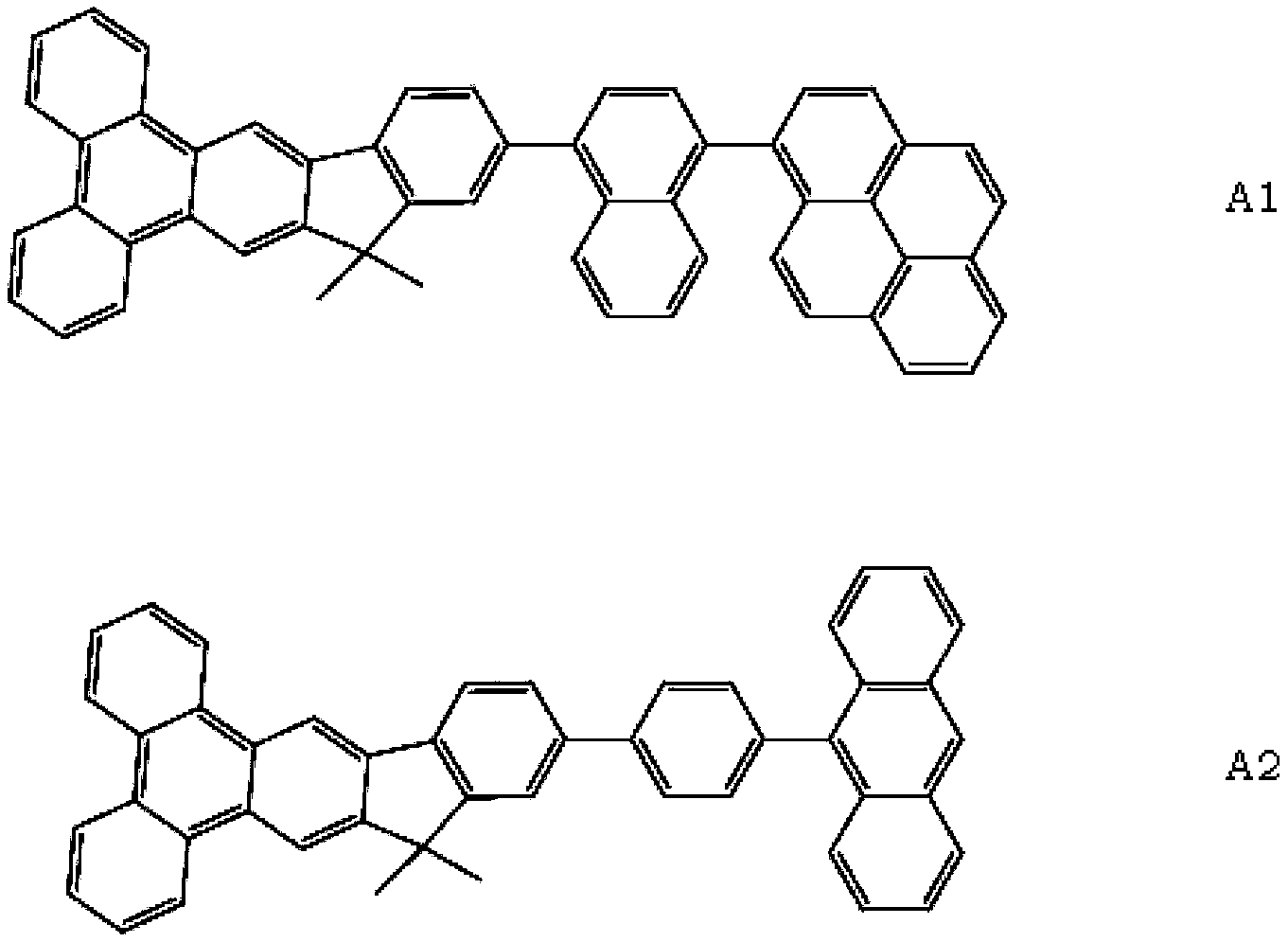
Indene benzophenanthrene derivative and organic light-emitting device using same
A technology of indenatriphenylene and derivatives is applied in the fields of indenatriphenylene derivatives and organic electroluminescence devices, and can solve problems such as incapability of industrial application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of compound A12
[0046] Synthesis of 2-(diphenyl-2-yl)-7-bromo-9,9-dimethyl-9H-fluorene
[0047]
[0048] 35.2g (100mmol) 2,7-dibromo-9,9-dimethyl-9H-fluorene, 21.8g (110mmol) diphenyl-2-ylboronic acid, 2.31g (2mmol) tetrakis (triphenylphosphine ) Palladium, 75ml 2M Na 2 CO 3 , A mixture of 150ml EtOH and 300ml toluene was degassed and placed under nitrogen, then heated at 100°C for 12h. After the reaction was complete, the mixture was cooled to room temperature. The organic layer was extracted with ethyl acetate and water, dried over anhydrous magnesium sulfate, the solvent was removed, and the residue was purified by silica gel column chromatography (hexane-dichloromethane) to obtain a white solid product (26.8g, 63.0mmol, 63%). 1 H NMR (CDCl 3 ,400MHz): chemical shift (ppm)7.61(d,J=7.8Hz,1H),7.55~7.53(m,2H),7.49~7.42(m,5H),7.29(d,J=8.0Hz,1H) ,7.20~7.14(m,5H),6.98(s,1H),1.21(s,6H)
[0049] Synthesis of 12-bromo-10,10-dimethyl-10H-indeno[1,2-b]triphe...
Embodiment 2
[0065] Synthesis of Compound A13
[0066] Synthesis of 1-bromo-6-(naphthalen-1-yl)pyrene
[0067]
[0068] 27.2g (75.6mmol) 1,6-dibromopyrene, 13g (75.6mmol) naphthalene-1-yl boronic acid, 0.87g (0.756mmol) tetrakis (triphenylphosphine) palladium, 57ml 2M Na 2 CO 3 A mixture of , 120 ml EtOH and 300 ml toluene was degassed and placed under nitrogen, then heated at 90° C. for 24 h. After the reaction was complete, the mixture was cooled to room temperature. Then 500 ml of MeOH were added with stirring, and the precipitated product was filtered off with suction. Recrystallization from 1,4-dioxane gave 14 g of a brown product (yield 45.5%).
[0069] Synthesis of 10,10-Dimethyl-12-(6-(naphthalen-1-yl)pyren-1-yl)-10H-indeno[1,2-b]triphenylene
[0070]
[0071] 7g (17.2mmol) of 1-bromo-6-(naphthalene-1-yl)pyrene, 8.1g (17.2mmol) of 2-(10,10-dimethyl-10H-indeno[1,2-b]benzene Phenanthrene-12-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 0.2g (0.172mmol) tetrakis(triphenylph...
Embodiment 3
[0073] Synthesis of Compound A15
[0074] Synthesis of 9-bromo-10-(naphthalene-2-yl)anthracene
[0075]
[0076] 15g (44.6mmol) 9,10-dibromoanthracene, 7.7g (44.6mmol) naphthalene-2-ylboronic acid, 0.52g (0.446mmol) tetrakis (triphenylphosphine) palladium, 33ml2M Na 2 CO 3 A mixture of , 60 ml EtOH and 150 ml toluene was degassed and placed under nitrogen, then heated at 90° C. for 24 h. After the reaction was complete, the mixture was cooled to room temperature. The organic layer was extracted with ethyl acetate and water, dried over anhydrous magnesium sulfate, the solvent was removed, and the residue was purified by silica gel column chromatography (hexane-dichloromethane) to obtain 10.4 g (61%) of a yellow solid product.
[0077] Synthesis of 10,10-Dimethyl-12-(10-(naphthalene-2-yl)anthracen-9-yl)-10H-indeno[1,2-b]triphenylene
[0078]
[0079] 6.6g (17.2mmol) 9-bromo-10-(naphthalene-2-yl) anthracene, 8.1g (17.2mmol) 2-(10,10-dimethyl-10H-indeno[1,2-b] Triphenylen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com