Fusion protein of Exendin-4 tandem polypeptide and human serum albumin, preparation and application thereof
A technology of human serum albumin and fusion protein, which is applied in the field of genetically engineered protein drugs, can solve the problems of prolonging the action time and achieve the effect of not easy to lose, easy to enlarge, and mature fermentation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Construction of fusion protein expression plasmid
[0039] The E-L1-E-L2 gene was synthesized by Shanghai Bioengineering Technology Service Company and cloned in the pBluescript SK plasmid, where E is Exendin-4, L1 is [Gly-Gly-Gly-Gly-Ser]2, and L2 is Gly-Gly -Gly-Gly-Ser. The sequence is as follows:
[0040] 5' CTC GAG AAA AGA CAT GGA GAG GGA ACC TTC ACC TCC GAC TTG TCC AAA CAA ATG
[0041] GAG GAG GAG GCC GTC CGT CTT TTC ATC GAG TGG CTG AAA AAT GGA GGA CCT TCC
[0042] TCC GGA GCC CCT CCT CCT TCC GGT GGT GGT GGA TCT GGT GGT GGT GGA TCT CAT GGT
[0043] GAA GGT ACT TTT ACT TCT GAT TTG TCT AAA CAA ATG GAA GAA GAA GCT GTT AGA TTG
[0044] TTT ATT GAA TGG TTG AAG AAC GGT GGT CCA TCT TCT GGT GCT CCA CCA CCA TCT GGT
[0045] GGT GGT GGA TCC 3'
[0046] There are XhoI restriction site and BamHI restriction site at its 5' end and 3' end respectively.
[0047]In order to secrete and express E-L1-E-L2 and HSA in the form of fusion protein in Pichia pa...
Embodiment 2
[0049] Embodiment 2: the expression of fusion protein
[0050] Referring to the Pichia expression kit, inoculate the positive single clone expressing the fusion protein engineered bacteria into 100ml BMGY culture medium, culture on a shaker at 30°C / 230rpm overnight to make OD 600 Reach 4-8. The bacteria were collected by centrifugation at 3000rpm / 2 minutes, the bacteria were resuspended in 50ml of BMMY culture medium, and cultured on a shaker at 30°C / 230rpm. Methanol was added every 24 hours to a final concentration of 0.7%, induced continuously for 3 days, centrifuged at 5000 rpm / 10 minutes, and the supernatant was harvested and stored at -30°C.
Embodiment 3
[0051] Embodiment 3: the purification of fusion protein
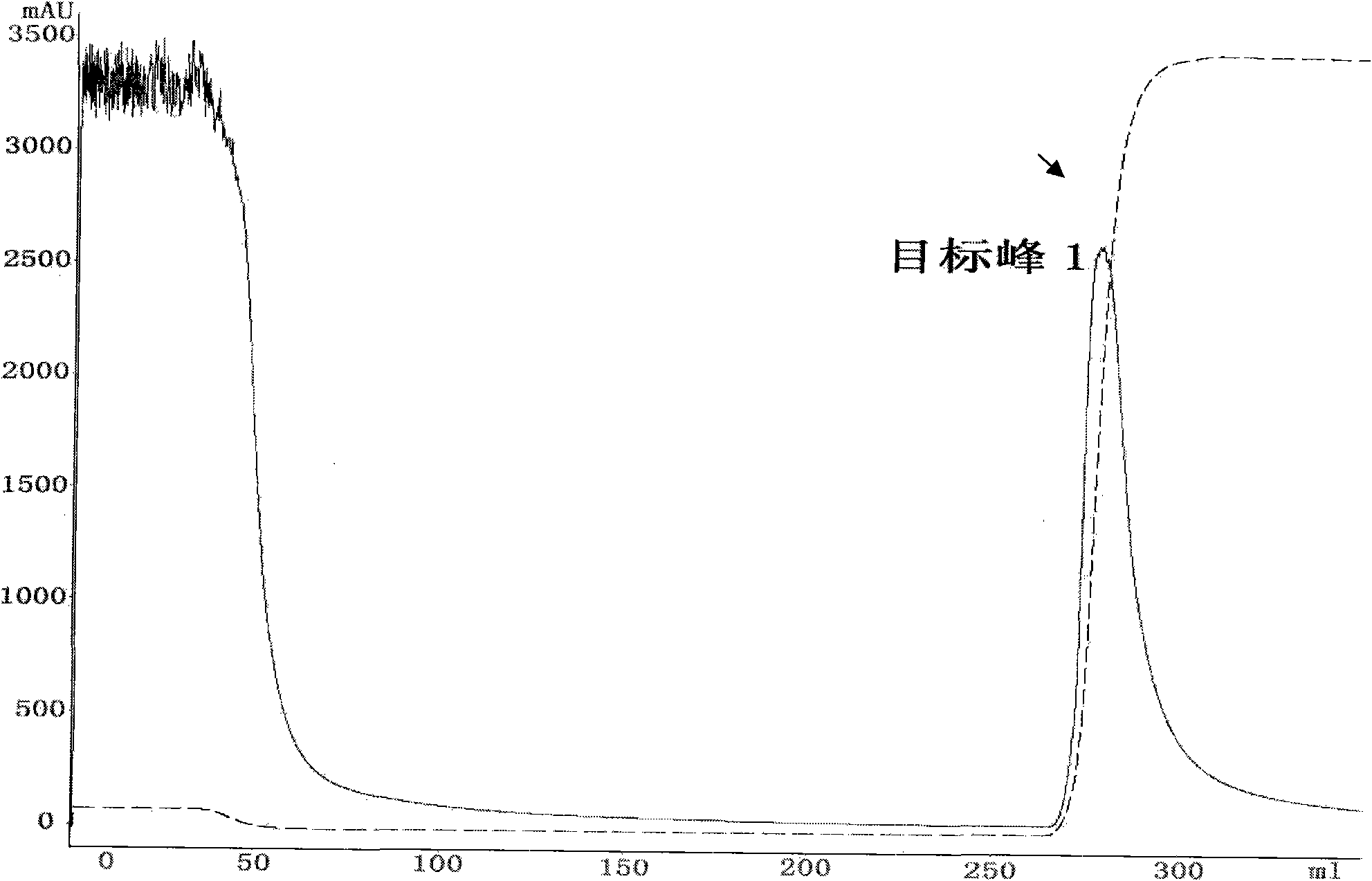
[0052] According to the Pichia fermented liquid obtained in Example 2, use double-distilled water to dilute to make its conductance equivalent to that of the affinity balance buffer, and adjust the pH to 6.0-7.0. Dilute the diluted fermented liquid with a 0.45 μm micropore Membrane filtration, then concentrated to a small volume using an ultrafiltration system with a molecular weight cut-off of 10,000; then at a flow rate of 8ml / min, use 20mM sodium phosphate buffer (pH7.0) as the mobile phase, pass through a Blue Sepharose 6 FastFlow column (GE health) to adsorb the fusion protein, and finally eluted with 20mM sodium phosphate and 2M sodium chloride (pH7.0) buffer, then collected the elution peak, and the detection wavelength was set at 215nm, where the target peak 1 was the expression containing the fusion protein product( figure 1 ).
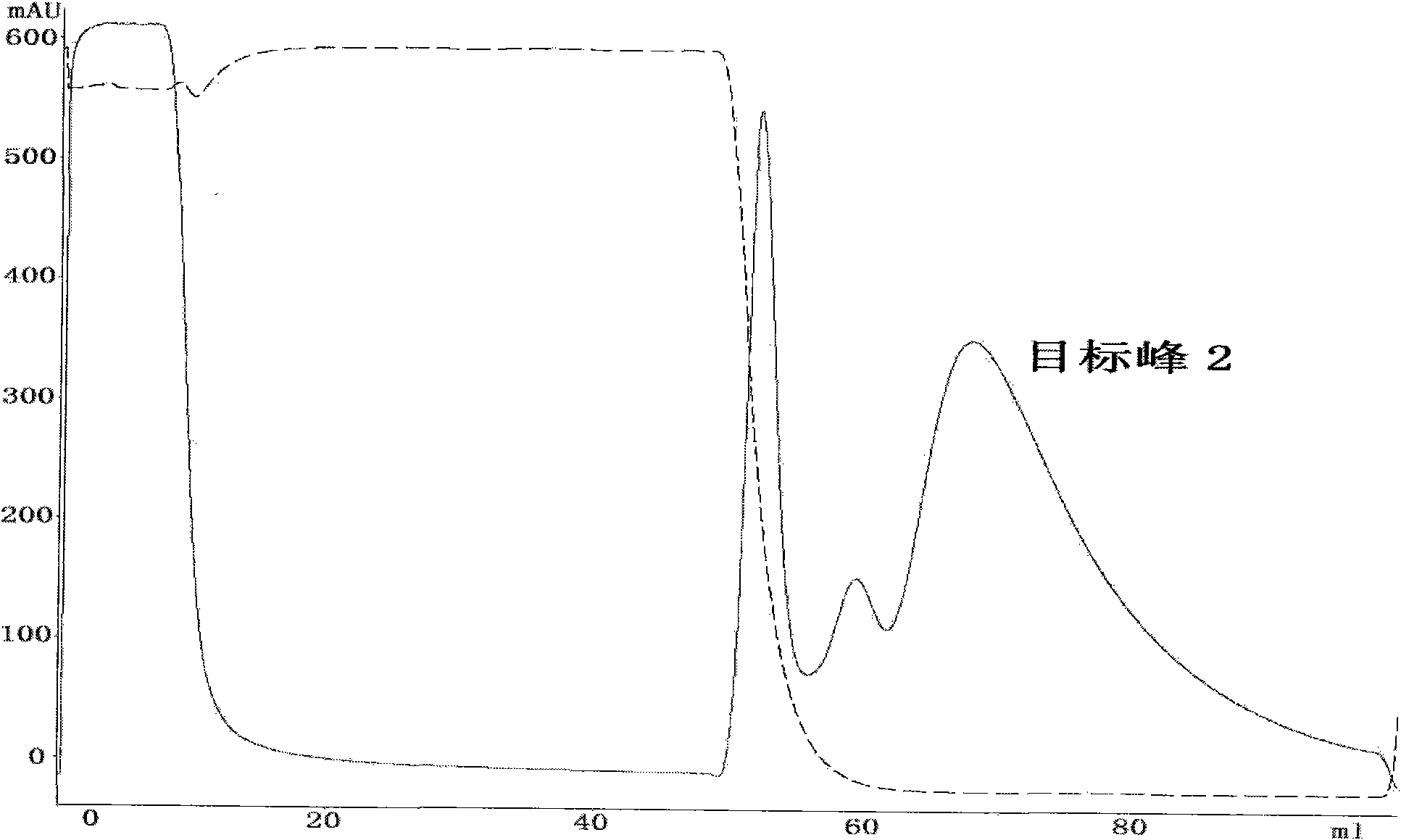
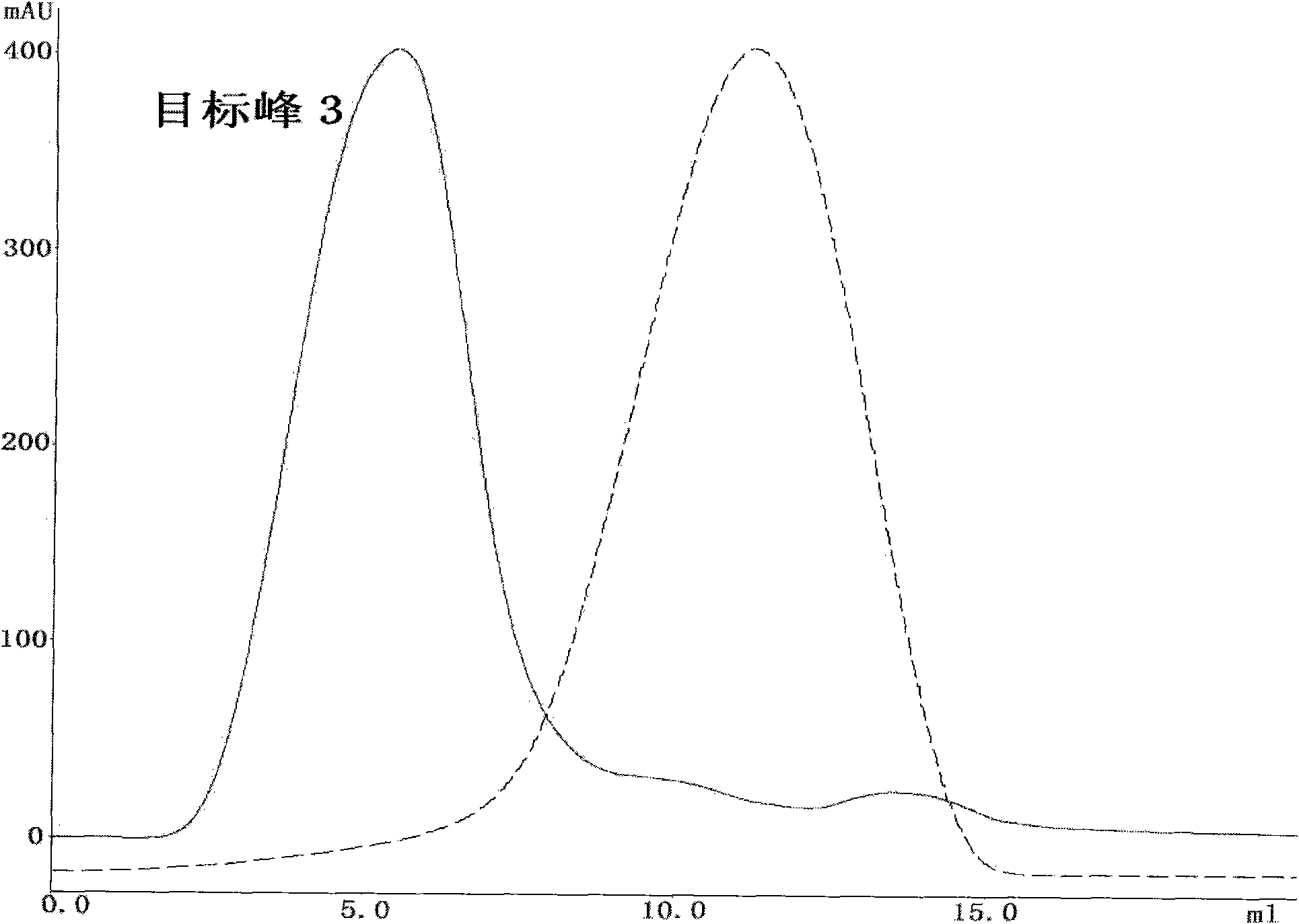
[0053] Collect the target peak 1 sample, add 20mM sodium phosphate and 2M ammon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com