Method for preparing magnesium hydroxide flame retardant by microchannel precipitation-hydrothermal process
A technology of magnesium hydroxide and flame retardant, applied in the direction of magnesium hydroxide, etc., to achieve the effects of controllable particle size, reduction of system energy consumption and complexity, and continuous reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
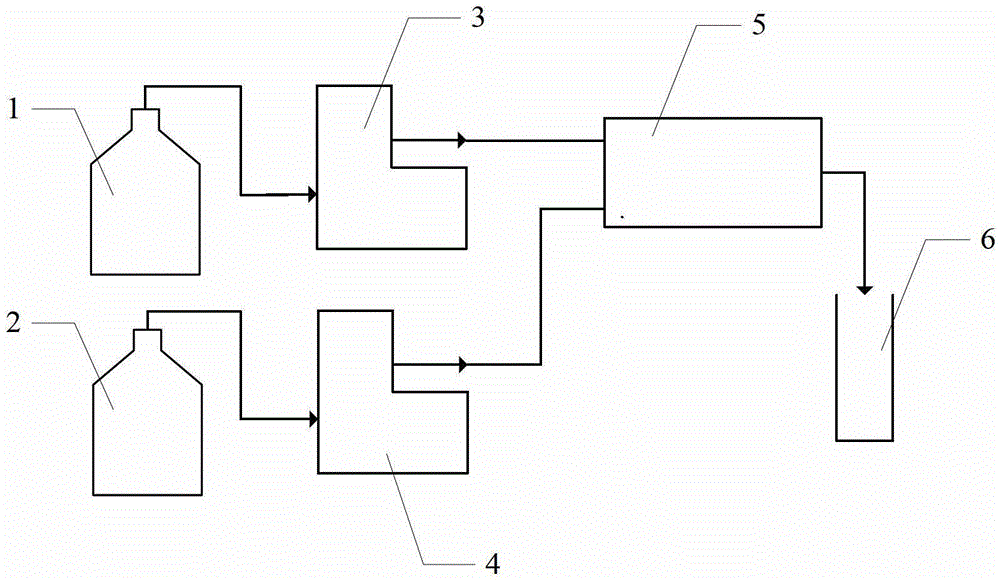
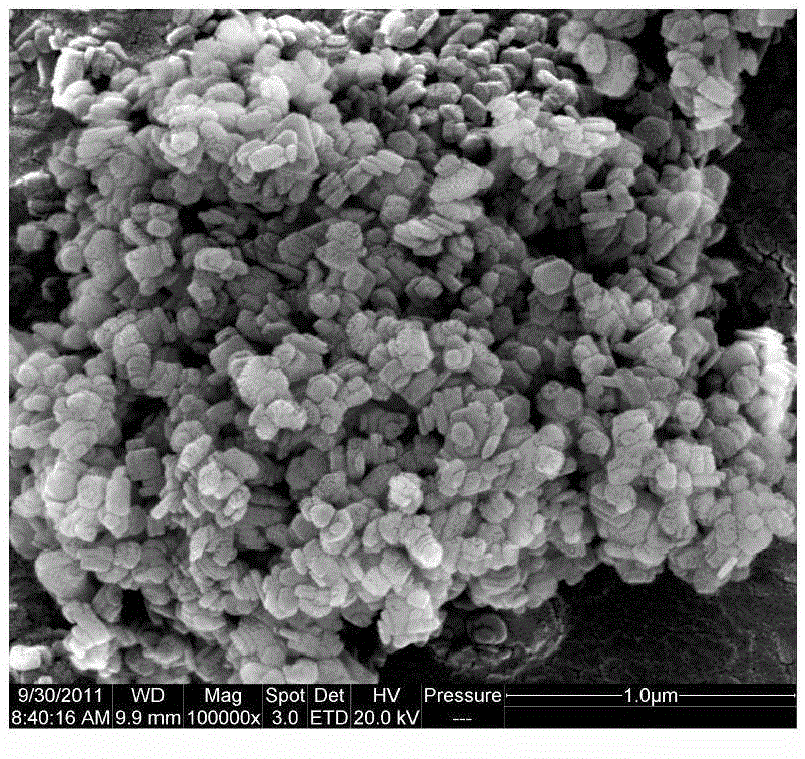
[0033] A 1.0 mol / L magnesium chloride solution and a 1.0 mol / L sodium hydroxide solution were prepared, and the two raw material solutions were respectively pumped to the microchannel reactor at room temperature. Wherein the pump speed of magnesium chloride solution is controlled at 50ml / min, and the pump speed of sodium hydroxide solution is controlled at 110ml / min, and the precipitation reaction mol ratio n (Mg 2+ ) / n(OH - ) is 1:2.2. The slurry at the outlet of the microchannel reactor directly enters the hydrothermal synthesis kettle, the hydrothermal reaction temperature is 150° C., and the hydrothermal time is 2 hours. The product after hydroheating is cooled, filtered, washed (detected by silver nitrate until no chloride ion is detected), dried at 110°C for 10 hours, and crushed to obtain a particle size of 100-200nm, a purity of 99.1%, and a specific surface area of 17.6m 2 / g of hexagonal flake magnesium hydroxide. The SEM picture of the product is attached fig...
Embodiment 2
[0035]A 2.0 mol / L magnesium chloride solution and a 2.0 mol / L sodium hydroxide solution were prepared, and the two raw material solutions were respectively pumped to the microchannel reactor at room temperature through advection pumping. Wherein the pump speed of magnesium chloride solution is controlled at 20ml / min, and the pump speed of sodium hydroxide solution is controlled at 50ml / min, and the precipitation reaction mol ratio n (Mg 2+ ) / n(OH - ) is 1:2.5. The slurry at the outlet of the microchannel reactor directly enters the hydrothermal synthesis kettle, the hydrothermal reaction temperature is 160° C., and the hydrothermal time is 4 hours. The product after hydroheating is cooled, filtered, washed (detected by silver nitrate until no chloride ion is detected), dried at 110°C for 10 hours, and crushed to obtain a particle size of 200-300nm, a purity of 98.6%, and a specific surface area of 13.2m 2 / g of hexagonal flake magnesium hydroxide. The SEM picture of the p...
Embodiment 3
[0037] A 3.0 mol / L magnesium chloride solution and a 6.0 mol / L sodium hydroxide solution were prepared, and the two raw material solutions were respectively pumped to the microchannel reactor at room temperature. Wherein the pump speed of magnesium chloride solution is controlled at 160ml / min, and the pump speed of sodium hydroxide solution is controlled at 120ml / min, and the precipitation reaction mol ratio n (Mg 2+ ) / n(OH - ) is 1:1.5. The slurry at the outlet of the microchannel reactor directly enters the hydrothermal synthesis kettle, the hydrothermal reaction temperature is 180° C., and the hydrothermal time is 4 hours. The product after hydroheating is cooled, filtered, washed (detected by silver nitrate until no chloride ion is detected), dried at 110°C for 10 hours, and crushed to obtain a particle size of 400-600nm, a purity of 99.0%, and a specific surface area of 11.8m 2 / g of hexagonal flake magnesium hydroxide. The SEM picture of the product is attached Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com