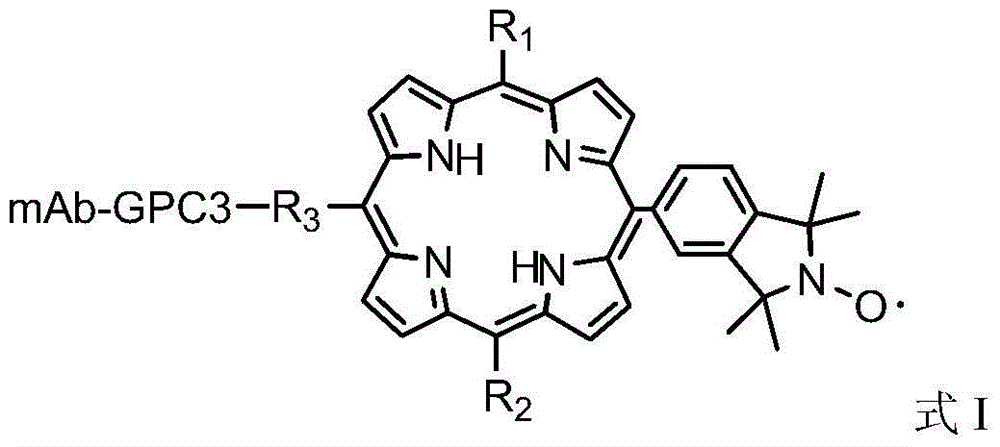
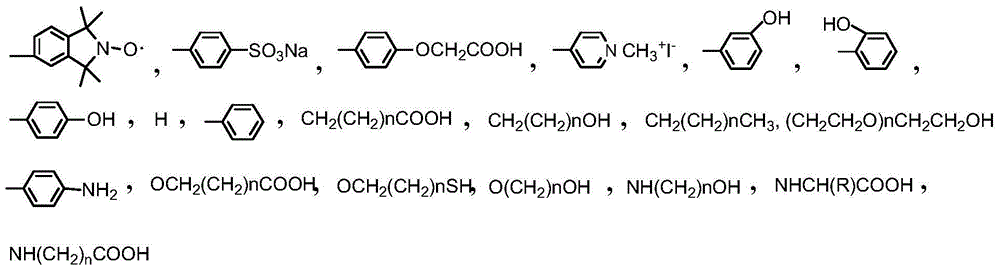Antibody labeled porphyrin iso-indole free radical compound and synthetic method thereof
A porphyrin isonitrogen and antibody labeling technology, applied in the fields of medicine and chemistry, can solve the problems of no tissue or organ selectivity or targeting, the influence of animal physiology and pathological processes, and fast metabolism, and achieve a small dosage. , low cost, long imaging time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 0.0476mg m-maleimide benzoyl-N-N-hydroxysuccinimide ester (1.515×10 -4 mmol, MBS, MW314.25) was added to 0.143 mg 5-(1',1',3',3'-tetramethyl-isozaindene-2'-nitrogen oxide radical)-15-aminophenyl -10,20-bis(sodium sulfonate phenyl)porphyrin (1.515×10 -4 mmol, MW947) was dissolved in 1 mL of phosphate buffer (0.01 mol / L, pH7.4), and vortexed at room temperature for 4 hours. Add 1 mg GPC3 antibody (66kDa, 1.515×10-5mmol) and vortex shake at room temperature for 4 hours. The reaction solution was dialyzed against ultra-pure water for 12 hours to obtain an antibody-labeled porphyrin isoazepine radical compound solution.
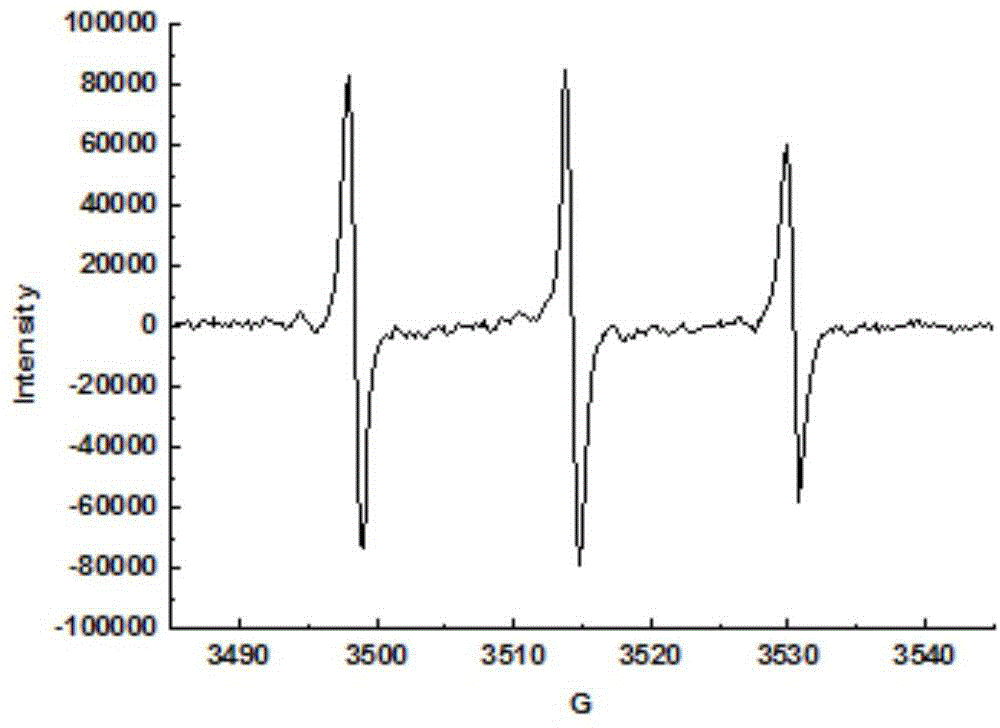
[0040] X-band EPR spectrum: The antibody-labeled porphyrin isoazepine free radical compound of Example 1 was dissolved in twice distilled water to form a dilute solution, and its electron paramagnetic resonance performance was measured on a Brucker EPR A300 electron paramagnetic resonance instrument. attached figure 1 It can be seen that the antibody-la...
Embodiment 2
[0042]0.0514mg N[ε-maleimidocaproic acid]hydrazide trifluoroacetic acid (1.515×10-4mmol, EMCH, MW339.27) and 0.029mg 1-ethyl-3-[3-dimethylaminopropyl Base] carbodiimide hydrochloride (1.515×10-4mmol, EDC, MW191.70) was added to 0.15mg of 5,10-bis(1',1',3',3'-tetramethyl-isozaindene -2'-nitrogen oxide radical)-15,20-bis(p-acetoxy)porphyrin (1.515×10-4mmol, MW990) dissolved in 1mL of acetate buffer (0.01mol / L,pH4.5) vortexed at room temperature for 4 hours. The solvent was evaporated to dryness under reduced pressure, and 1 mg of GPC3 antibody (66 kDa, 1.515×10-5 mmol) was added to the solid in 1 mL of phosphate buffer (0.01 mol / L, pH 7.4), and vortexed at room temperature for 4 hours. The reaction solution was dialyzed against ultra-pure water for 12 hours to obtain an antibody-labeled porphyrin isoazepine radical compound solution.
Embodiment 3
[0044] 0.029mg 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (1.515×10-4mmol, EDC, MW191.70) was added to 0.172mg 5-(1',1',3 ',3'-Tetramethyl-isozaindene-2'-nitrogen oxide radical)-10,15-bis(p-iodomethanepyridyl)-20-(p-butanoylpyridyl)-porphyrin (1.515×10-4mmol, MW1138) was dissolved in 1mL of acetate buffer (0.01mol / L, pH4.5), and vortexed at room temperature for 4 hours. The solvent was evaporated to dryness under reduced pressure, and 1 mg of GPC3 antibody (66 kDa, 1.515×10-5 mmol) was added to the solid in 1 mL of phosphate buffer (0.01 mol / L, pH 7.4), and vortexed at room temperature for 4 hours. The reaction solution was dialyzed against phosphate buffer for 12 hours to obtain an antibody-labeled porphyrinindene free radical compound solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com