Method for promoting efficient water-soluble expression of arginine deiminase
An arginine deiminase and water-soluble technology, which is applied in the biological field to achieve the effect of promoting high-efficiency water-soluble expression and overcoming the insoluble expression of arginine deiminase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
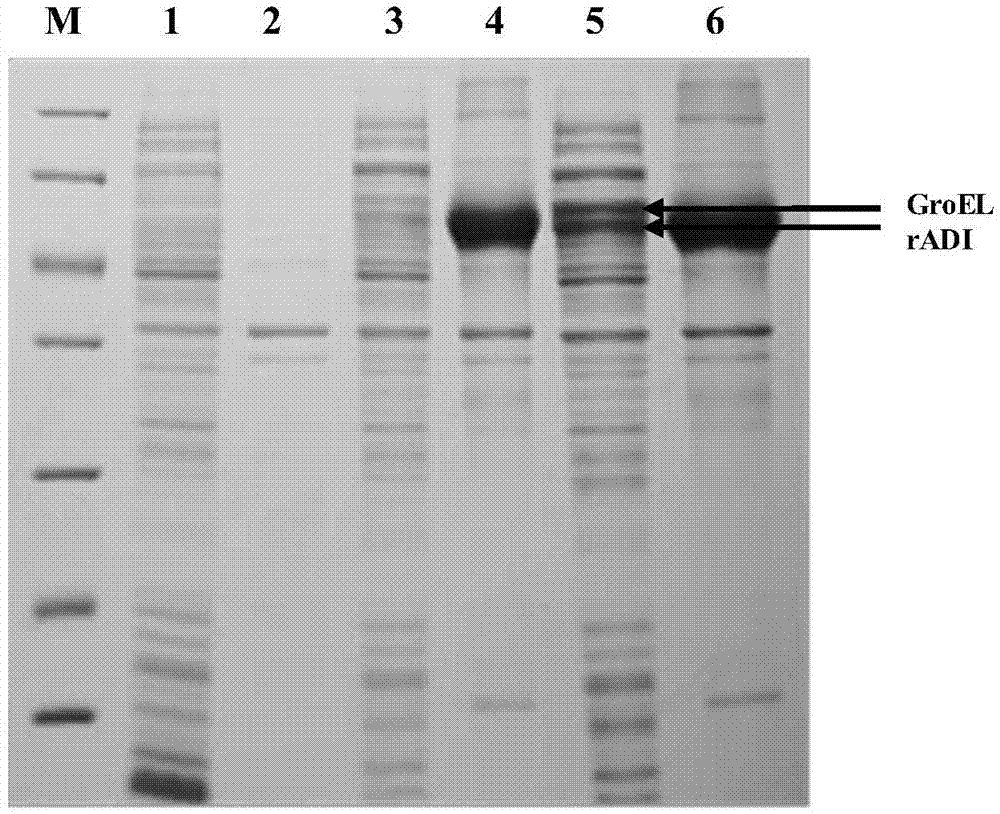
[0026] Example 1 Arginine deiminase derived from Pseudomonas putida was constructed as a recombinant expression vector and expressed heterologously in Escherichia coli.
[0027] (1) Arginine deiminase (GenBank accession no.P41142) derived from Pseudomonas putida was used according to the codon frequency table of the expression host E.coliBL21(DE3), using the "codon random distribution" Optimize the strategy to fully artificially synthesize the ADI gene. Then, using the PCR method, the corresponding restriction endonucleases NcoI and HindIII were cloned into the upstream and downstream of the ADI gene, and connected to the pET-30a-c(+) vector containing the 6His tag to construct the recombinant expression vector pET30a- ADI, and co-transformation with the pGro7 plasmid encoding the molecular chaperone GroEL / GroES into E.coliBL21(DE3) to obtain engineering bacteria containing double plasmids (pET30a-ADI and pGro7).
[0028] The "codon random distribution" strategy refers to weigh...
Embodiment 2
[0042] The method is the same as in Example 1, except that the concentration of D-glucose in step (3) is 1g / L, the final concentration of L-arabinose in step (3) is 0.2g / L, and the concentration of L-arabinose in step (4) The concentration of arginine was 1 g / L.
Embodiment 3
[0044] The method is the same as in Example 1, except that the concentration of D-glucose in step (3) is 10g / L, the final concentration of L-arabinose in step (3) is 0.8g / L, and the concentration of L-arabinose in step (4) Arginine concentration is 10g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com