Method for purifying type 71 enterovirus
A technology of enterovirus and purification method, which is applied in the field of purification of enterovirus 71 (EV71), can solve the problems of high cost and residual additives, and achieve the effect of easy process, good safety, and no contamination by exogenous factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Centrifugal clarification: select a suitable centrifuge and rotor according to the volume of the cell culture, centrifuge at 4°C for 30 minutes with a centrifugal force of 9000g, and combine the centrifuged supernatant, which is the virus clarification solution;
[0038] 2) Concentration by ultrafiltration
[0039] Use 0.5M sodium hydroxide (NaOH) in the ultrafiltration system equipped with an appropriate number (depending on the number of samples to be processed) of 100KD ~ 500KD membrane packs at an appropriate pressure (inlet pressure 20 ~ 30psi, return pressure 8psi, through mouth pressure 0psi) and flow rate (1-3LPM) for 60 minutes, then wash the system with 5 times the volume of water for injection to remove residual NaOH, and then wash with PBS until the pH value is neutral; The ultrafiltration system concentrates to 1 / 30-1 / 50 of the original volume, washes and filters 2-3 times, and finally obtains the EV71 virus concentrate.
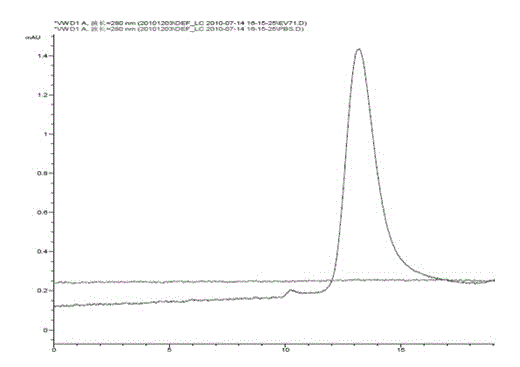
[0040] 3) Gel filtration chrom...
Embodiment 2
[0052] 1) Filtration clarification: The preferred method is to pre-filter through a surface filter with a pore size of 2.5 μM; then filter again through a filter with a pore size of 0.45 μM. The filtrate is the clarified liquid of EV71 virus.
[0053] 2) Concentration by ultrafiltration
[0054] Use 0.5M sodium hydroxide (NaOH) in the ultrafiltration system equipped with an appropriate number (depending on the number of samples to be processed) of 100KD ~ 500KD membrane packs at an appropriate pressure (inlet pressure 20 ~ 30psi, return pressure 8psi, through mouth pressure 0psi) and flow rate (1-3LPM) for 60 minutes, then wash the system with 5 times the volume of water for injection to remove residual NaOH, and then wash with PBS until the pH value is neutral; The ultrafiltration system concentrates to 1 / 30-1 / 50 of the original volume, washes and filters 2-3 times, and finally obtains the EV71 virus concentrate.
[0055] 3) Gel filtration chromatography
[0056] Put Sepha...
Embodiment 3
[0069] Various tests of EV71 virus purification solution:
[0070] The inactivated vaccine prepared by the EV71 virus purification solution of the present invention has been tested for the following indicators: free formaldehyde residue, bovine serum albumin residue, host cell protein residue, host cell DNA residue, purity, antigen identification test, bacterial endotoxin residue, Antibiotic Residue, Sterility Test, Abnormal Toxicity Check Inactivation Verification and pH Check. The verification method refers to the relevant method in the appendix of "Chinese Pharmacopoeia" (2010 edition). The test results are shown in Table 1.
[0071] Table I
[0072] Test items Verification standard test result Free formaldehyde residue ≤50ug / dose 6.5ug / dose bovine serum albumin residue ≤50ng / dose ≤30ng / dose host cell protein residue ≤50ug / dose ≤30ug / dose host cell DNA residue ≤100pg / dose ≤10pg / dose purity ≥95% ≥95% antigen ide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com