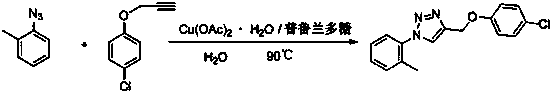Method for preparing 1, 2, 3-triazole compound
A technology of azide compounds and compounds, which is applied in the field of catalytic synthesis of organic matter, can solve the problems of not conforming to the concept of green production, high ligand toxicity, large amount of copper application, etc., to overcome the high toxicity of ligands, simple catalytic system, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
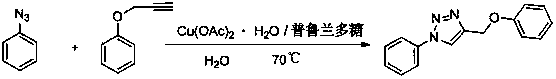
[0010] 4-Benzyloxy-1-phenyl-1H-1,2,3-triazole
[0011]
[0012] Weigh copper acetate (1mmol) and pullulan (10mmol), dissolve completely in water, and add propargyl phenyl ether (100mmol) and phenyl azide (100mmol) at a temperature of 70°C under stirring conditions, Keep the temperature until the end of the reaction. After cooling, a solid precipitates out. Filter and purify to obtain 4-benzyloxy-1-phenyl-1H-1,2,3-triazole.
Embodiment 2
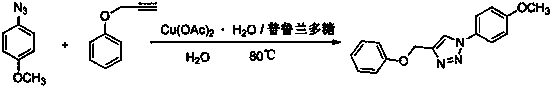
[0014] 4-Benzyloxy-1-p-methoxyphenyl-1H-1,2,3-triazole
[0015]
[0016] Weigh copper acetate (1mmol) and pullulan (1mmol), dissolve completely in water, and add propargyl phenyl ether (100mmol) and p-methoxyphenyl azide at a temperature of 80°C under stirring conditions (100mmol), keep the temperature until the end of the reaction, after cooling, a solid precipitates out, filters and purifies to obtain 4-benzyloxy-1-p-methoxyphenyl-1H-1,2,3-triazol azole.
Embodiment 3
[0018] 4-(4-Chloro-benzyloxy)-1-o-tolyl-1H-1,2,3-triazole
[0019]
[0020] Weigh copper acetate (1mmol) and pullulan (0.1mmol), dissolve completely in water, and add propargyl p-chlorophenyl ether (100mmol) and o-methylphenyl Azide (100mmol), keep the temperature until the end of the reaction, after cooling, solids precipitate out, filter and purify to obtain 4-(4-chloro-benzyloxy)-1-o-tolyl-1H-1,2 ,3-triazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com