Method for preparing diindolyl methane derivatives
A technology of bisindolylmethane and derivatives, which is applied in the field of preparation of bisindolylmethane derivatives, can solve the problems of harsh reaction conditions, difficult to obtain raw materials, long reaction time and the like, and achieves good substrate applicability and low cost of catalysts. , the effect of efficient response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
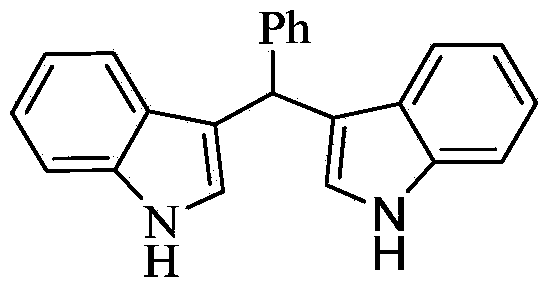
[0014] Taking the preparation of the following formula compound bis-indolephenylmethane as an example, the raw materials used and the preparation method thereof are as follows:
[0015]
[0016] 0.106g (1.0mmol) benzaldehyde, 0.234g (2.0mmol) indole, 0.0125g (0.05mmol) titanocene dichloride, 0.0145g (0.1mmol) 8-hydroxyquinoline, 0.0093g (0.1mmol) aniline Add 5 mL of acetonitrile, react at 50°C for 1 hour, stop the reaction, add 2 mL of saturated aqueous sodium bicarbonate solution to the system, extract three times with 10 mL of ethyl acetate, combine the organic phases, dry with anhydrous magnesium sulfate, remove the solvent, and wash with ethyl acetate The mixture of ester and n-hexane with a volume ratio of 9:1 was separated by mobile phase column chromatography to obtain bis-indolephenylmethane as a bright pink solid with a yield of 95%.
[0017] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the charact...
Embodiment 2
[0023] Taking the preparation of the following formula compound bisindole p-methoxyphenylmethane as an example, the raw materials used and the preparation method thereof are as follows:
[0024]
[0025] In Example 1, the benzaldehyde used is replaced by equimolar p-methoxybenzaldehyde, aniline is replaced by equimolar pyridine, and other steps are the same as in Example 1 to prepare pink solid bisindole p-methoxybenzene Methyl methane, its productive rate is 86%, and characterization data is: 1 H NMR (400MHz, CDCl 3 )δppm: 7.89(s, 2H), 7.37(dd, J=16.7, 7.9Hz, 4H), 7.25(m, 2H), 7.16(t, J=7.2Hz, 2H), 7.01(d, J=7.0 Hz, 2H), 6.82(m, 2H), 6.65(s, 2H), 5.84(s, 1H), 3.78(d, J=2.1Hz, 3H); 13 C NMR (101MHz, CDCl 3 ) δppm: 157.93, 136.73, 136.23, 129.60, 127.10, 123.49, 121.90, 120.11, 120.00, 119.20, 113.58, 110.98, 55.21, 39.36.
Embodiment 3
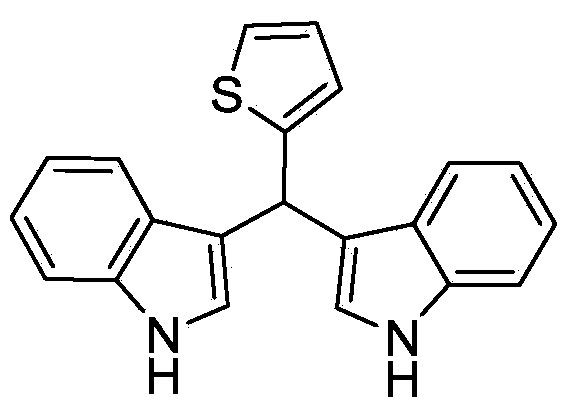
[0027] Taking the preparation of the following formula compound bisindole-α-thiophene methane as an example, the raw materials used and its preparation method are as follows:
[0028]
[0029] In Example 1, the benzaldehyde used is replaced with equimolar α-thiophene formaldehyde, and aniline is replaced with equimolar pyridine. Other steps are the same as in Example 1, and pink solid bisindole-α-thiophene methane is prepared. The yield is 81%, and the characterization data are: 1 H NMR (400MHz, CDCl 3 )δppm: 6.09 (s, 1H), 6.87-7.37 (m, 11Ar-H), 10.49 (s, 2H, NH); 13 C NMR (101MHz, CDCl 3 ) δppm: 149.35, 136.61, 126.37, 126.13, 124.55, 123.26, 120.95, 118.30, 118.25, 111.37, 35.14.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com