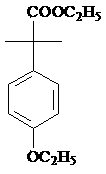
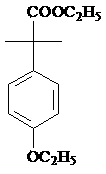
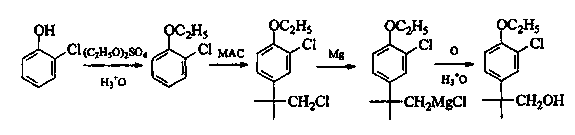
Preparation method of 2-(4-oxethyl phenyl)-2-methyl propanol
An ethoxyphenyl, methyl propanol technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of complex hydrolysis products, low dechlorination yield and high requirements, and achieves Reduced risk, environmentally friendly, process-reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, a preparation method of 2-(4-ethoxyphenyl)-2-methylpropanol, using borohydride / metal salt mixed system as reducing agent for 2-(4-ethoxybenzene base)-2-methylpropanoic acid methyl ester is reduced to obtain 2-(4-ethoxyphenyl)-2-methylpropanol;
[0038] The specific restoration method is as follows:
[0039] Take 1 mole of ester and dissolve it in 300-600ml of alcohol solvent, or in a mixed solvent composed of alcohol solvent and water or ether solvent, the alcohol solvent is methanol or ethanol, and the ether solvent is selected from tetrahydrofuran, 2- Methyl tetrahydrofuran, dioxane, diethyl ether, isopropyl ether or methyl tert-butyl ether; the esters described are:
[0040]
[0041] Raise the temperature to 30°C; add borohydride and metal salt, the molar ratio of borohydride, metal salt to substrate is 1.5:0.5:1; continue to keep warm for 3 hours; stir and add hydrochloric acid dropwise until the pH of the reaction material is 7 -8, then add 200-500m...
Embodiment 2
[0043] Embodiment 2, a kind of preparation method of 2-(4-ethoxyphenyl)-2-methyl propanol, use borohydride / metal salt mixed system as reducing agent pair 2-(4-ethoxybenzene base)-2-methylpropanoic acid methyl ester is reduced to obtain 2-(4-ethoxyphenyl)-2-methylpropanol;
[0044] The specific restoration method is as follows:
[0045] Take 1 mole of ester and dissolve it in 300-600ml of alcohol solvent, or in a mixed solvent composed of alcohol solvent and water or ether solvent, the alcohol solvent is methanol or ethanol, and the ether solvent is selected from tetrahydrofuran, 2- Methyl tetrahydrofuran, dioxane, diethyl ether, isopropyl ether or methyl tert-butyl ether; the esters described are:
[0046]
[0047] Raise the temperature to 85°C; add borohydride and metal salt, the molar ratio of borohydride, metal salt and substrate is 4.0: 2.0:1; continue to keep warm for 8 hours; stir and add hydrochloric acid dropwise until the pH of the reaction material is 7 -8, then...
Embodiment 3
[0049] Example 3, a preparation method of 2-(4-ethoxyphenyl)-2-methylpropanol, using borohydride / metal salt mixed system as reducing agent for 2-(4-ethoxybenzene base)-2-methylpropanoic acid methyl ester is reduced to obtain 2-(4-ethoxyphenyl)-2-methylpropanol;
[0050] The specific restoration method is as follows:
[0051] Take 1 mole of ester and dissolve it in 300-600ml of alcohol solvent, or in a mixed solvent composed of alcohol solvent and water or ether solvent, the alcohol solvent is methanol or ethanol, and the ether solvent is selected from tetrahydrofuran, 2- Methyl tetrahydrofuran, dioxane, diethyl ether, isopropyl ether or methyl tert-butyl ether; the esters described are:
[0052]
[0053] Raise the temperature to 60-70°C; add borohydride and metal salt, the molar ratio of borohydride, metal salt to substrate is 2.0:1.2:1; continue to keep warm for 5-6 hours; stir and add hydrochloric acid dropwise to the reaction mass The pH value is 7-8, then add 200-500m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com