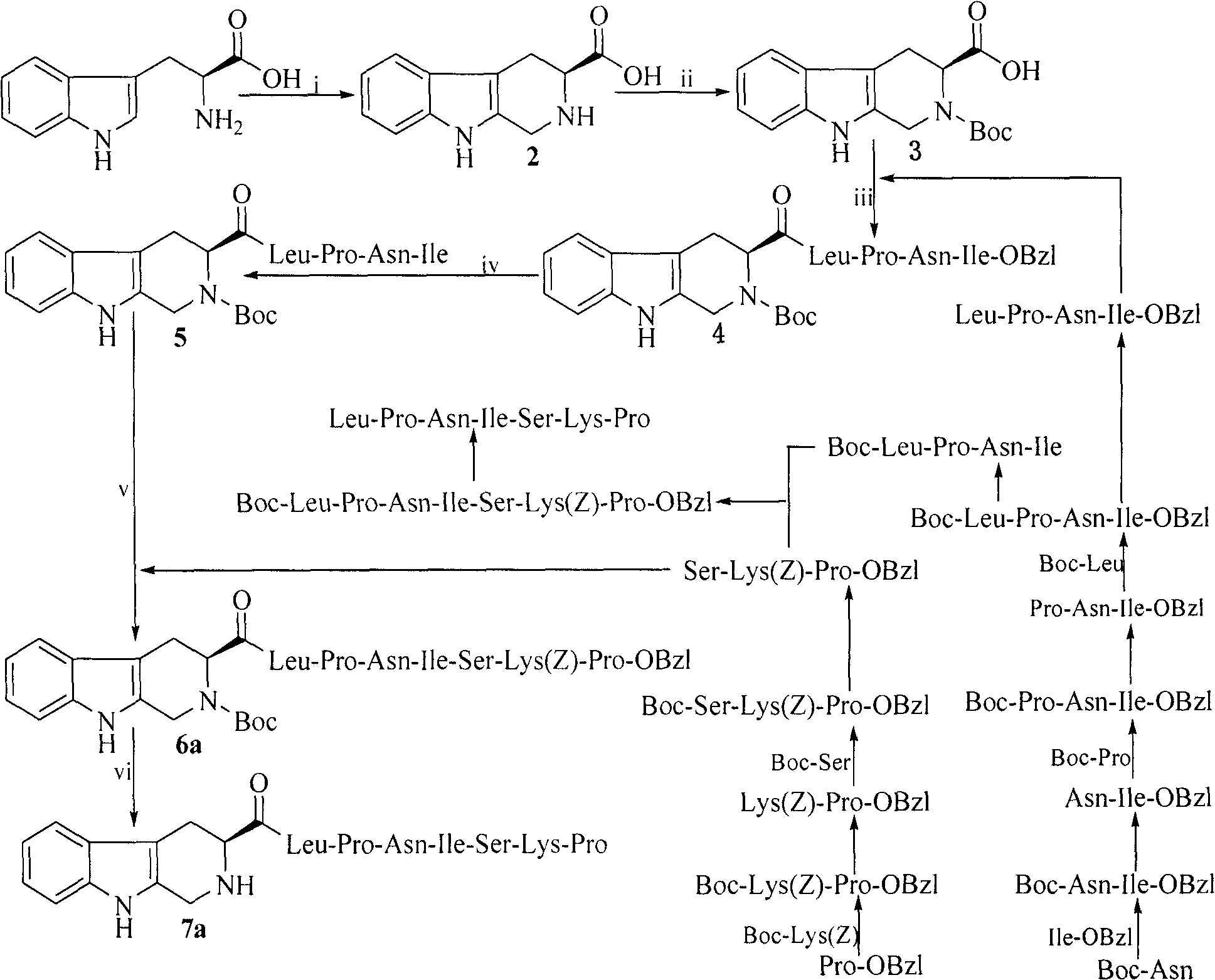
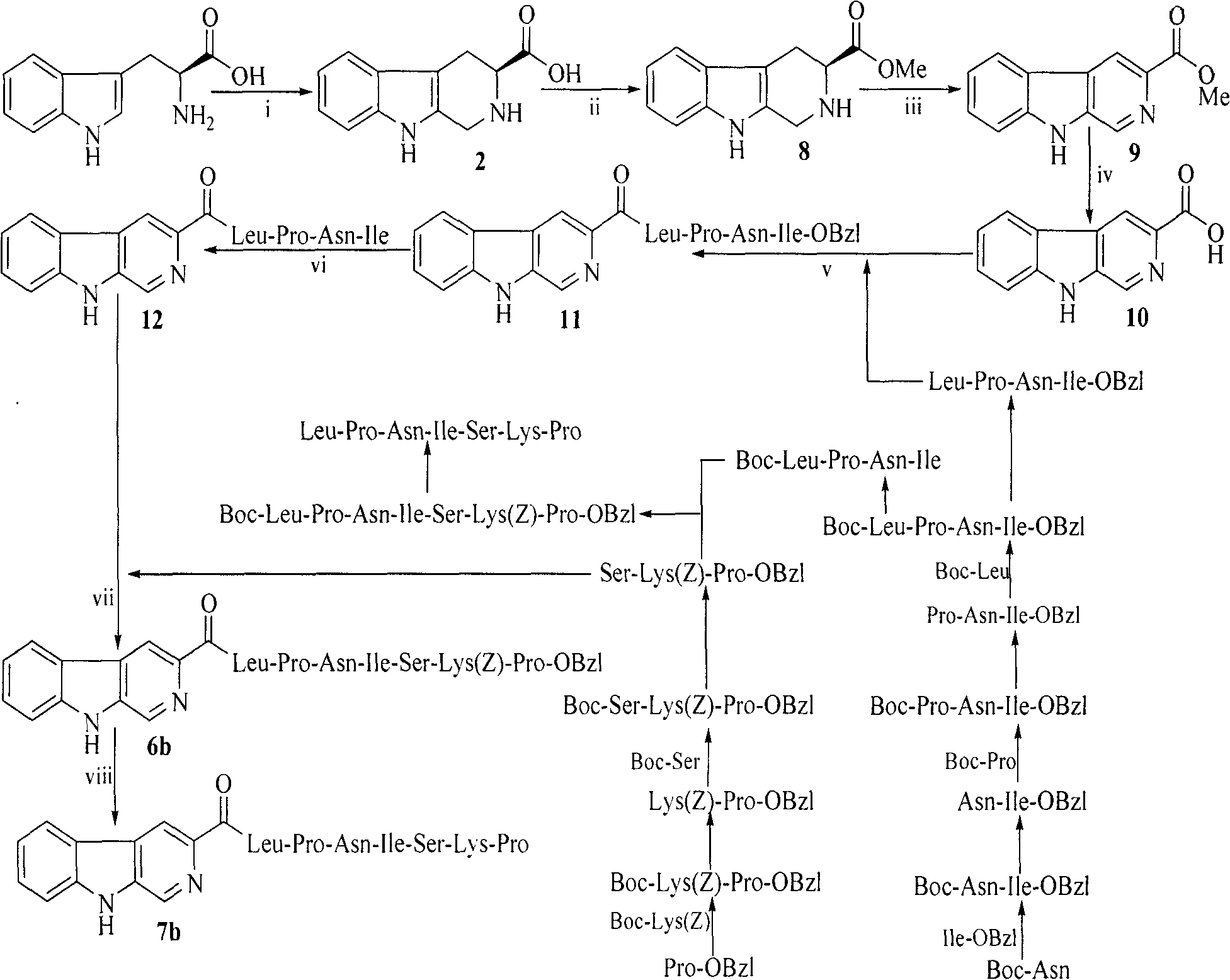
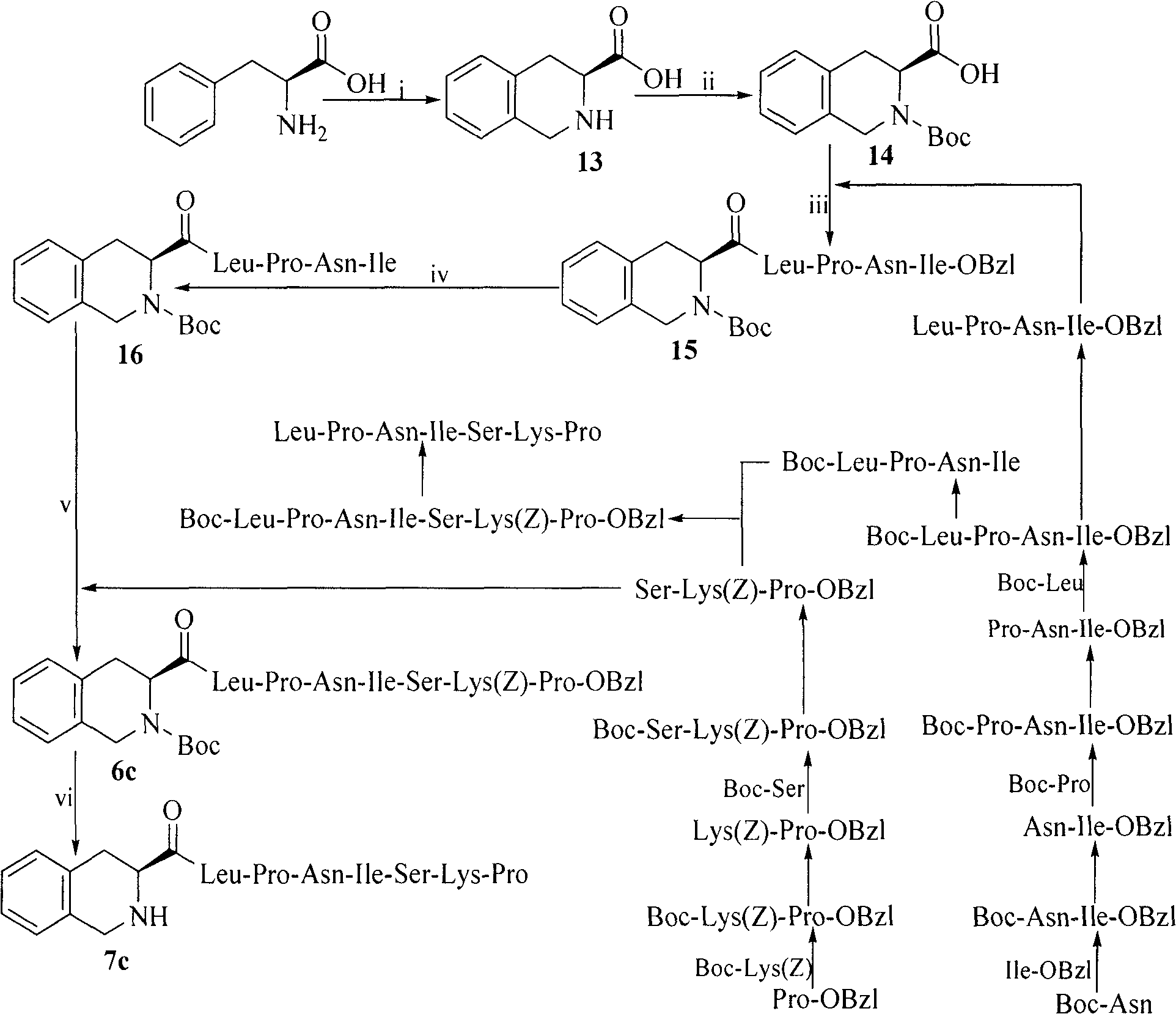
Heterocyclic carboxylic acid-modified anti-tumor oligopeptides and their synthesis, anti-tumor effects and application
A heterocyclic carboxylic acid, anti-tumor technology, applied in anti-tumor drugs, peptide/protein components, medical preparations containing active ingredients, etc., can solve the problems of unsatisfactory curative effect and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of Pro-OBzl
[0054] Place 50 mL of benzyl alcohol in a reaction flask cooled in an ice-salt bath and stir vigorously, 20 mL of SOCl 2 Slowly add it dropwise into the reaction flask containing methanol through a constant pressure separatory funnel, remove the ice-salt bath after stirring for 30 minutes, add 5.0g (43.6mmol) L-Pro to the reaction solution, react at room temperature for 48 hours, TLC board monitoring No When the starting point was reached, the reaction was completed. The solution was concentrated to dryness under reduced pressure, and the residue was dissolved in 8 mL of methanol, and then concentrated to dryness under reduced pressure. This operation was repeated at least 2 times. The residue was added with anhydrous diethyl ether, and the diethyl ether was fully contacted with the residue as far as possible, and then concentrated to dryness under reduced pressure. This operation was repeated 2 times. The residue was recrystallize...
Embodiment 2
[0055] Example 2 Preparation of Boc-Pro
[0056] Put 5.75g (50mmol) of L-Pro in a 250mL eggplant-shaped bottle, add 1mL of water to dissolve, and slowly add 25mL of NaOH aqueous solution (2N) dropwise under stirring in an ice bath to obtain solution (I). 13.08g (60mmol) (Boc) 2 O was placed in a 50mL small beaker, and 25mL of dioxane was added to dissolve to obtain solution (II). The solution (II) was added dropwise to the solution (I) under stirring in an ice bath, NaOH aqueous solution (2N) was slowly added dropwise under an ice bath to adjust the pH to 9, and the mixture was stirred under an ice bath. After 30 minutes, the pH of the reaction mixture was checked and adjusted to maintain a pH of 9 with aqueous NaOH (2N). Continue the reaction, adjust the pH value to 9 with NaOH aqueous solution (2N) at any time, and pump out the gas generated by the reaction, and monitor the reaction progress by TLC. The developing system is dichloromethane-methanol (20:1). About 48 hours...
Embodiment 3
[0057] Example 3 Preparation of Boc-Lys(Z)
[0058] Add 6.08g (24.34mmol) of anhydrous copper sulfate to the three-necked flask, add 10mL of distilled water, put it into an oil bath and heat to 100°C, reflux, and dissolve the anhydrous copper sulfate. Then add 5.34g L-Lys into the three-necked flask, and reflux in the oil bath for 2 hours. After the reaction, sodium bicarbonate was added under an ice bath to adjust the pH to 8, and then 10 g (40.2 mmol) of Bzl-OSu was added to react at room temperature for 8 hours. After the reaction, the reaction solution was filtered, and the filter cake was washed once with distilled water, acetone, and ether respectively. Then put the filter cake back into the three-necked flask, add EDTA and stir for 4 hours, filter, repeat the stirring, filter, and dry to obtain 20 g of L-Lys(Z), which is a colorless solid. The obtained 20g (71.4mmol) L-Lys (Z) and 18.69g (85.7mmol) (Boc) 2 O was reacted to afford 16.56 g (61%) of the title compound a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com