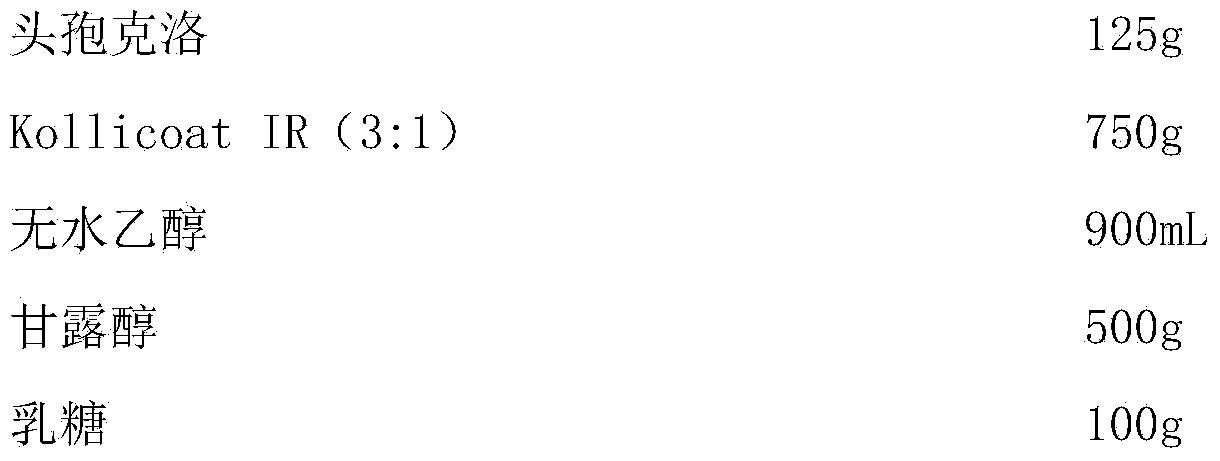Cefaclor capsule and preparation method thereof
A technology of Cefaclor capsules and Cefaclor, which is applied in the field of medicine and can solve problems such as delamination, unstable quality of cefaclor raw materials, and poor quality stability of cefaclor preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019]
[0020] Preparation Process:
[0021] Pass cefaclor through a 100 mesh sieve, dissolve Kollicoat IR (3:1) in absolute ethanol, add cefaclor, stir evenly and dry under reduced pressure to remove ethanol, mix the dried granules with sucrose passed through a 100 mesh sieve evenly , packed in capsules.
Embodiment 2
[0023]
[0024] Preparation Process:
[0025] Pass cefaclor through a 100 mesh sieve, dissolve Kollicoat IR (3:1) in absolute ethanol, add cefaclor, stir evenly and dry under reduced pressure to remove ethanol, mix the dried granules with mannitol, The lactose is mixed evenly, filled into capsules and packaged.
Embodiment 3
[0027]
[0028] Preparation Process:
[0029] Pass cefaclor through a 100-mesh sieve, dissolve Kollicoat IR (3:1) in absolute ethanol, add cefaclor, stir evenly and dry under reduced pressure to remove ethanol, mix the dried granules with mannitol and sucrose evenly, pour Capsule packaging. Comparative Example 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com