Hydrophobic association polymer containing capsaicin active monomer and preparation method thereof
A technology of hydrophobic association and active monomers, applied in drilling compositions, chemical instruments and methods, etc., can solve the problems of sudden changes in solution properties, poor thermal and biological stability, high cost, and achieve viscosity increase and The effect of strong salt resistance, favorable for industrial production and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
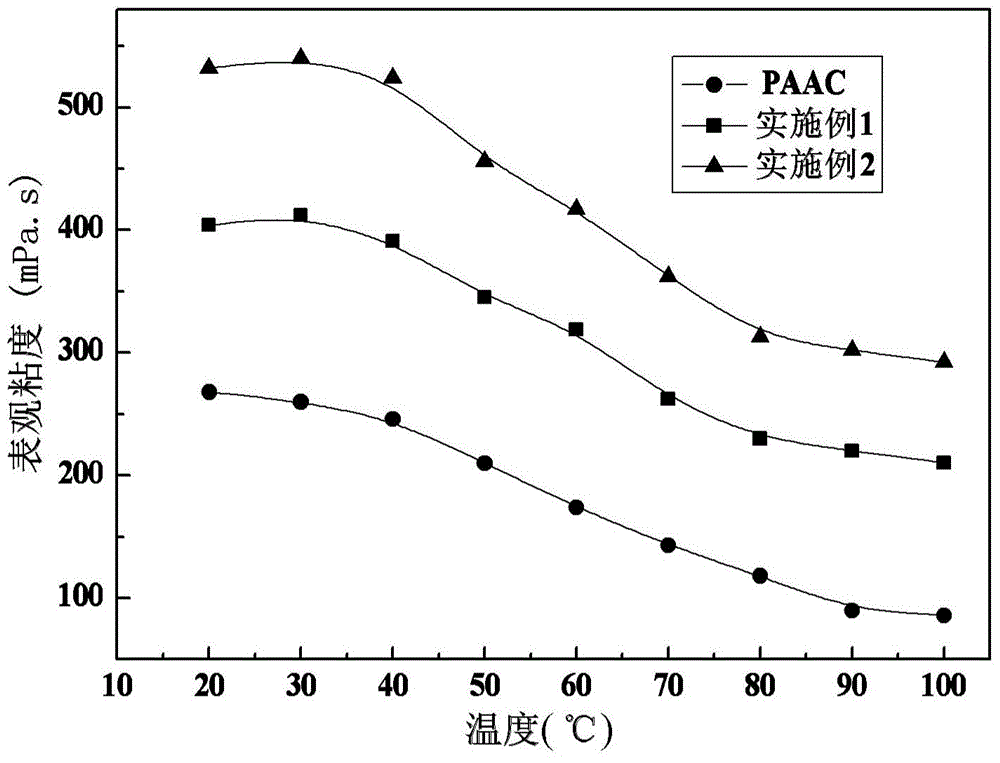
[0035] Preparation Example 1: Synthesis of Hydrophobic Association Polymer Containing Capsaicin Active Monomer
[0036] Dissolve 7.1g of acrylamide and 2.16g of acrylic acid in 40ml of distilled water, slowly add NaOH solution with a mass fraction of 10% to adjust the pH of the system to 6.4, add distilled water to control the total monomer concentration to 15% (mass fraction), and then add 2.35g of surfactant sodium dodecyl sulfate (SDS), after fully stirring, add 1.12g of N-(4-hydroxy-3-methoxy-benzyl) acrylamide, stirring continuously to fully dissolve in the SDS micelles , forming a nearly transparent homogeneous system. After purging nitrogen for 20 minutes to remove oxygen, 0.052 g of azobisisobutylamidine hydrochloride was added and reacted at a constant temperature of 56° C. for 8 hours to obtain a transparent polymer product with a certain viscosity. Precipitate with absolute ethanol, then cut the precipitate into small pieces with scissors, soak in absolute ethanol ...
preparation Embodiment 2
[0039] Preparation Example 2: Synthesis of Hydrophobic Association Polymer Containing Capsaicin Active Monomer
[0040] Dissolve 7.1 g of acrylamide and 2.16 g of 2-acrylamido-2-methylpropanesulfonic acid in 40 ml of distilled water, slowly add NaOH solution with a mass fraction of 10% to adjust the pH of the system to 6.4, and add distilled water to control the total amount of monomers. The concentration is 15% (mass fraction), then add 2.37g of surfactant sodium dodecyl sulfate (SDS), stir well and add 1.20g of N-(4-hydroxy-3-methylthio-benzyl)acrylamide, Stir continuously to make it fully dissolve in the SDS micelles to form a nearly transparent homogeneous system. After purging nitrogen for 20 minutes to remove oxygen, 0.052 g of azobisisobutylamidine hydrochloride was added and reacted at a constant temperature of 56° C. for 8 hours to obtain a transparent polymer product with a certain viscosity. Precipitate with absolute ethanol, then cut the precipitate into small pie...
preparation Embodiment 3
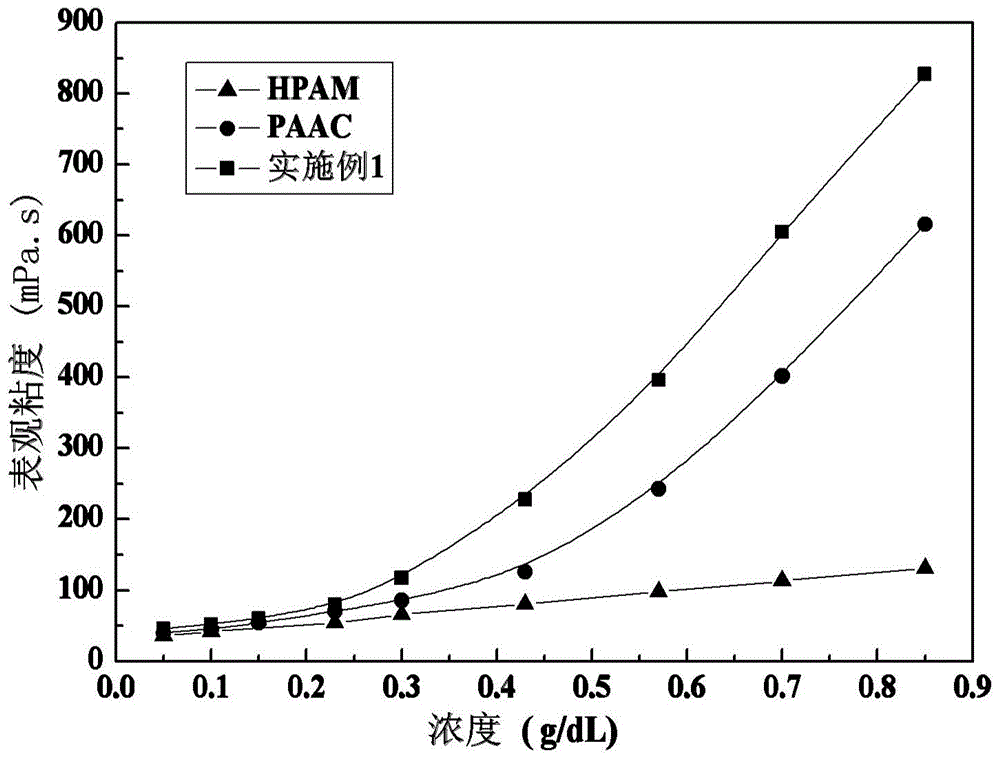
[0042] Control experiment 1: Synthesis of acrylamide-acrylic acid binary copolymer (hereinafter referred to as HPAM)
[0043] Dissolve 7.1 g of acrylamide and 2.16 g of acrylic acid in 50 ml of distilled water, slowly add dropwise a 10% NaOH solution to adjust the pH of the system to 6.4, add distilled water to control the total monomer concentration to 15% (mass fraction), and blow nitrogen Deoxidize for 20 minutes, add 0.046g of azobisisobutylamidine hydrochloride (0.5% of the total monomer mass), react at a constant temperature at 56°C for 8 hours, precipitate with absolute ethanol, and then cut the precipitate with scissors. into small pieces, soaked in absolute ethanol for 3 days, and dried under vacuum at 45°C to obtain the above-mentioned acrylamide-acrylic acid binary copolymer with an intrinsic viscosity of 670.1 mL / g.
[0044] Control experiment 2: Synthesis of acrylamide-acrylic acid-N-octylacrylamide copolymer (PAAC)
[0045] Dissolve 7.1g of acrylamide and 2.16g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com