Polypeptide Cbf-14 resisting infection of drug-resistant bacteria and application thereof
A technology of anti-drug-resistant bacteria and antibacterial peptides, applied in the field of biomedicine, can solve the problems of poor proteasome stability, high toxicity of antimicrobial peptides, and high production costs of antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
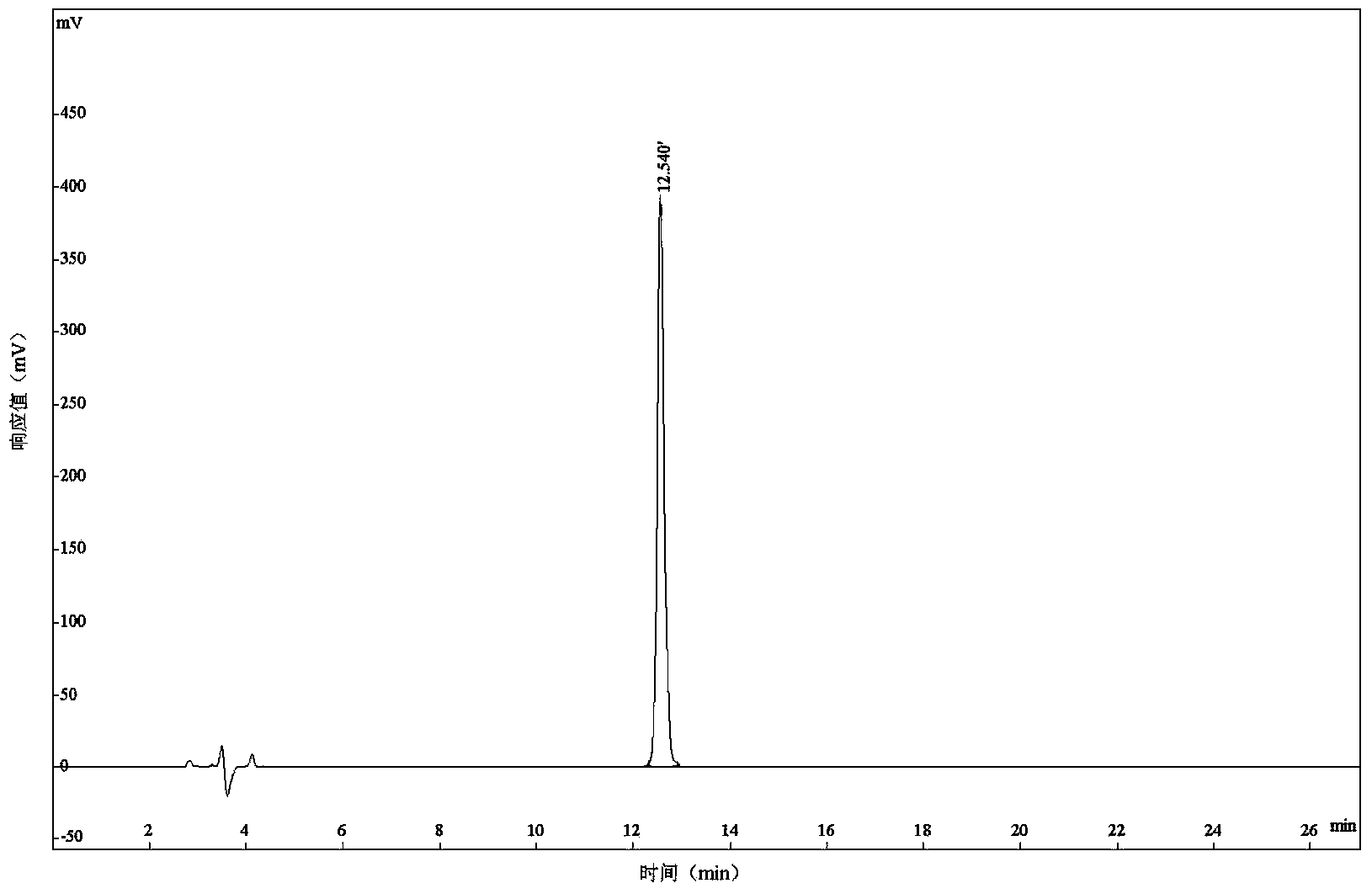
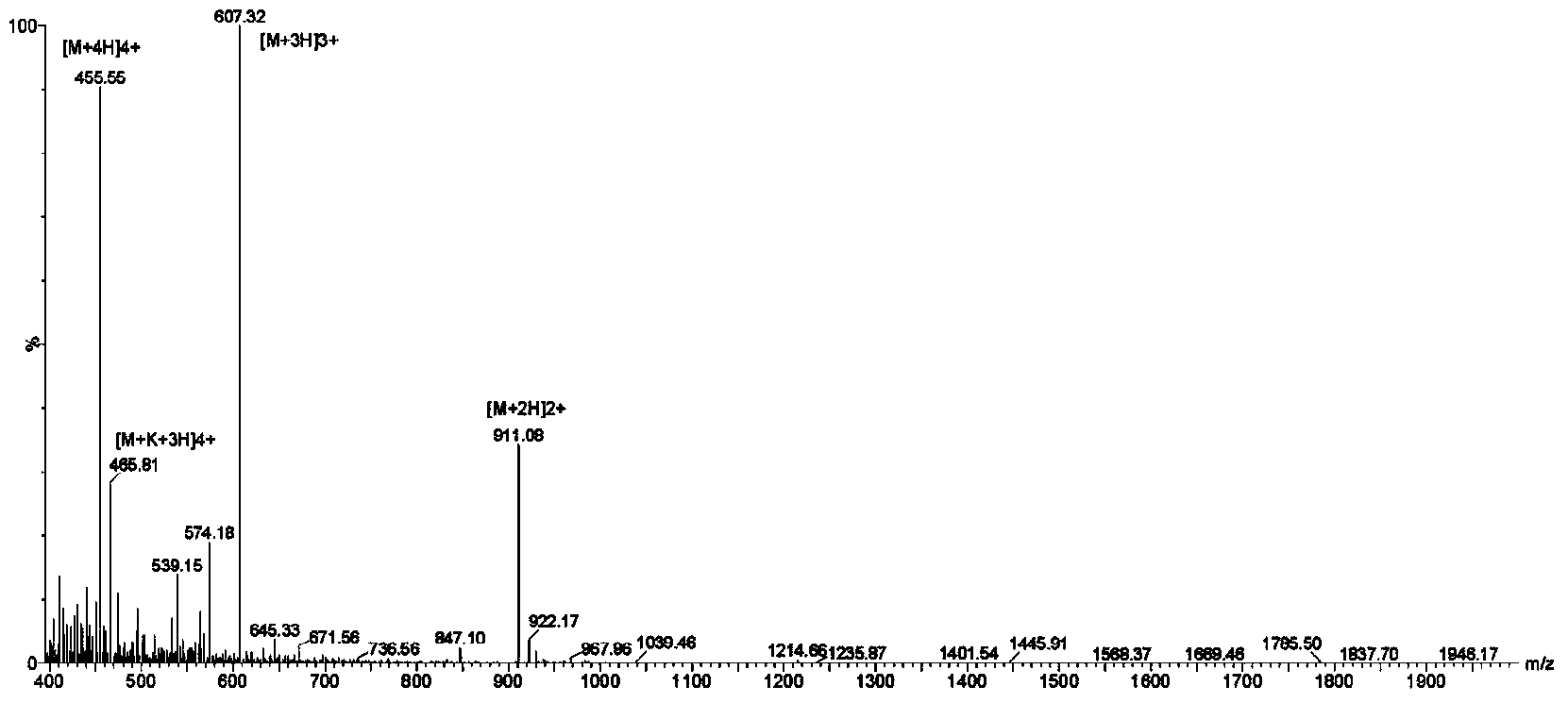
[0021] Synthesis and Structure Confirmation of Antibacterial Peptide Cbf-14:
[0022] The polypeptide of the present invention is artificially synthesized by conventional solid-phase synthesis method. The specific experimental steps are as follows:
[0023] The polypeptide sequence of the prepared Cbf-14 is:
[0024] H-Arg-Leu-Leu-Arg-Lys-Phe-Phe-Arg-Lys-Leu-Lys-Lys-Ser-Val-OH
[0025] Synthesis of peptides: The synthesis of peptides is carried out one by one from the C-terminal to the N-terminal. Soak Fmoc-Val-Wang Resin in dichloromethane for 15 minutes, wait for the resin to swell, and remove the dichloromethane; add hexahydropyridine / DMF solution with a volume ratio of 1:4, use nitrogen agitation, and react twice for After 5 minutes and 15 minutes, the resin was washed 9 times with DMF after the reaction. Take a small amount of resin and add 2-3 drops of each color test agent ABC (A solution: ninhydrin / absolute ethanol solution; B solution: pyridine; C solution: phenol...
Embodiment 2
[0034] The drug effect of polypeptide Cbf-14 of the present invention on penicillin-resistant bacteria:
[0035] (1) Preparation of inoculum
[0036] Connect the bacteria used in the experiment from the glycerol tube to the slant of nutrient agar, cultivate overnight at 37°C, then pick a little to inoculate in 2ml nutrient broth medium, culture at 37°C for 8h, and dilute to 100% with sterile MH broth culture solution. 5 Bacterial suspension around CFU / ml.
[0037] (2) Drug configuration
[0038] Accurately weigh Cbf-14 and penicillin to prepare a drug solution with a concentration of 1024 μg / ml, filter it through a 0.22 μm membrane aseptically, aliquot it, and store it at -70°C for future use.
[0039] (3) Determination of minimum inhibitory concentration (MIC)
[0040]Use sterile MH broth to dilute the drug stock solution to 1ml solution with drug concentration of 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 μg / ml. Take 1ml of the above bacterial liquid and add it to ...
Embodiment 3
[0060] The toxicity of the polypeptide antibacterial peptide Cbf-14 of the present invention includes the determination of hemolysis and the determination of toxicity to eukaryotic cells.
[0061] Hemolytic determination of the polypeptide antimicrobial peptide Cbf-14 of the present invention:
[0062] Fresh sheep whole blood was centrifuged at 3000 rpm for 10 min in a refrigerated centrifuge (4°C), and the obtained sheep red blood cells were washed three times with PBS and suspended at a specific gravity of 10% by volume. Mix the same volume of resuspended sheep red blood cells with the serially diluted drugs to make the final concentration of Cbf-14 to be 512, 256, 128, 64, 32, 16, 8, 4 and 2, 1 μg / ml, and set the PBS group as negative Control, 0.1% Triton X-100 group was used as positive control, and each gradient was set to 6 parallels. All mixtures were incubated in a 37°C incubator for 1 h. Then centrifuge at 3000rpm for 10min in a refrigerated centrifuge (4°C), take t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com