New erlotinib hydrochloride crystal form and preparation method thereof
A technology of erlotinib hydrochloride and crystal form, applied in the field of medicinal chemistry, can solve the problems of environmental hazards, unsuitable for industrial production, high price of trifluorotoluene, etc., and achieve the effects of low toxicity, good solubility and stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation method of new crystal form of erlotinib hydrochloride
[0036] Add 5.0 g of erlotinib base into a dry and clean 250 mL four-neck flask, add 75 mL of ethanol, heat to 40°C, and stir for 30 min. After the solid dissolves, heat filter to remove insoluble matter. Pass in dry HCl gas under slow stirring, stir for 0.5h; cool to 3±3°C under stirring, stir for 1h; filter, rinse with 5mL ethanol, and dry the filter cake in a blast oven at 35°C for 6h to obtain erlohydrochloride A new crystal form of tinib.
[0037] The product quality is 5.40g, the yield is 99%, and the HPLC purity is 99.7%.
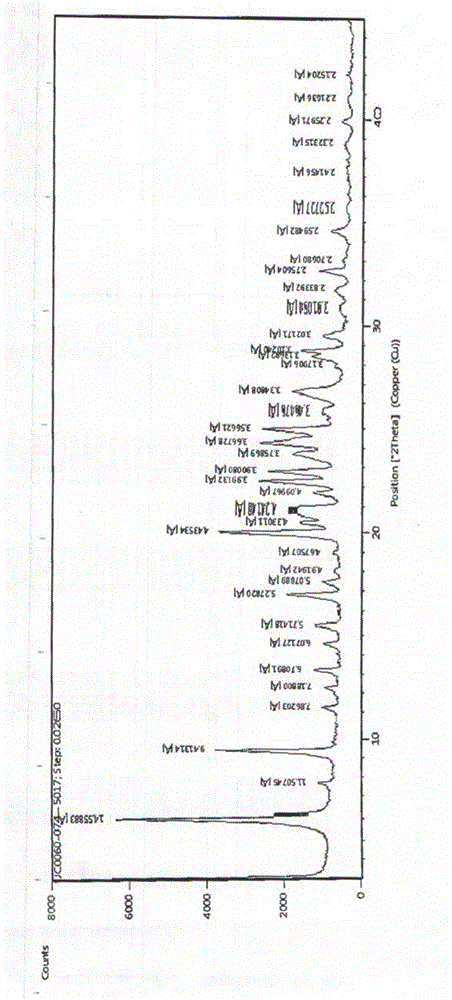
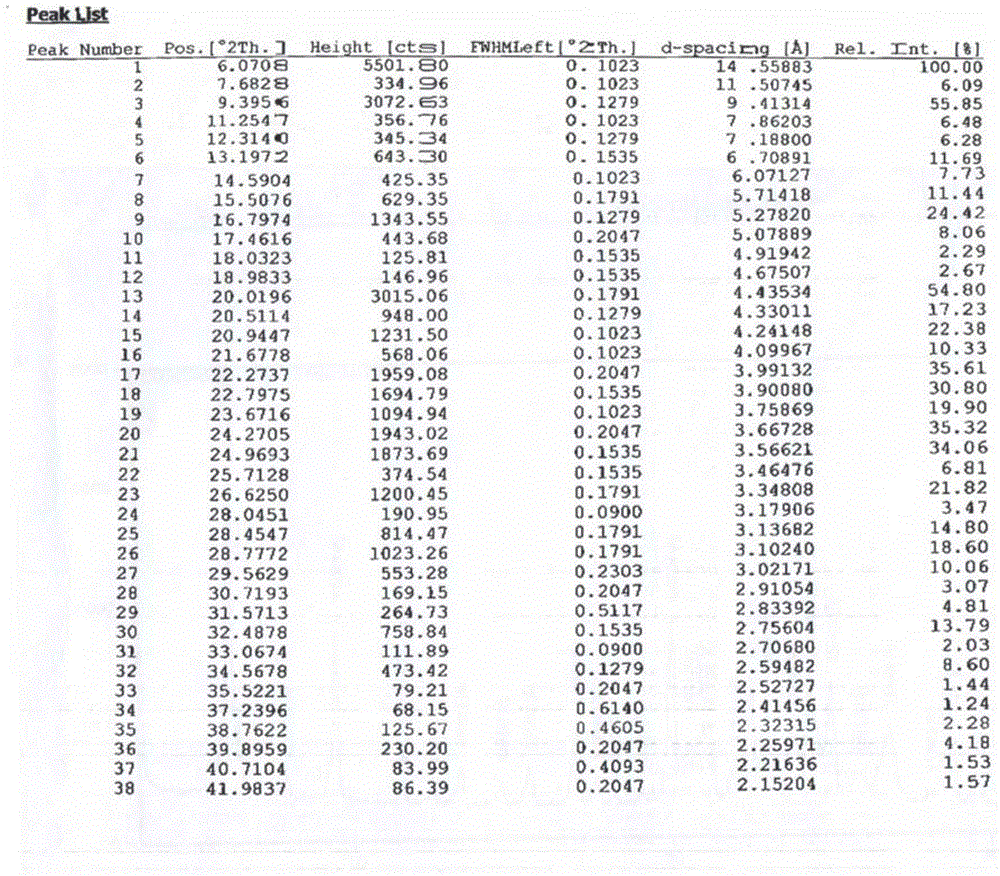
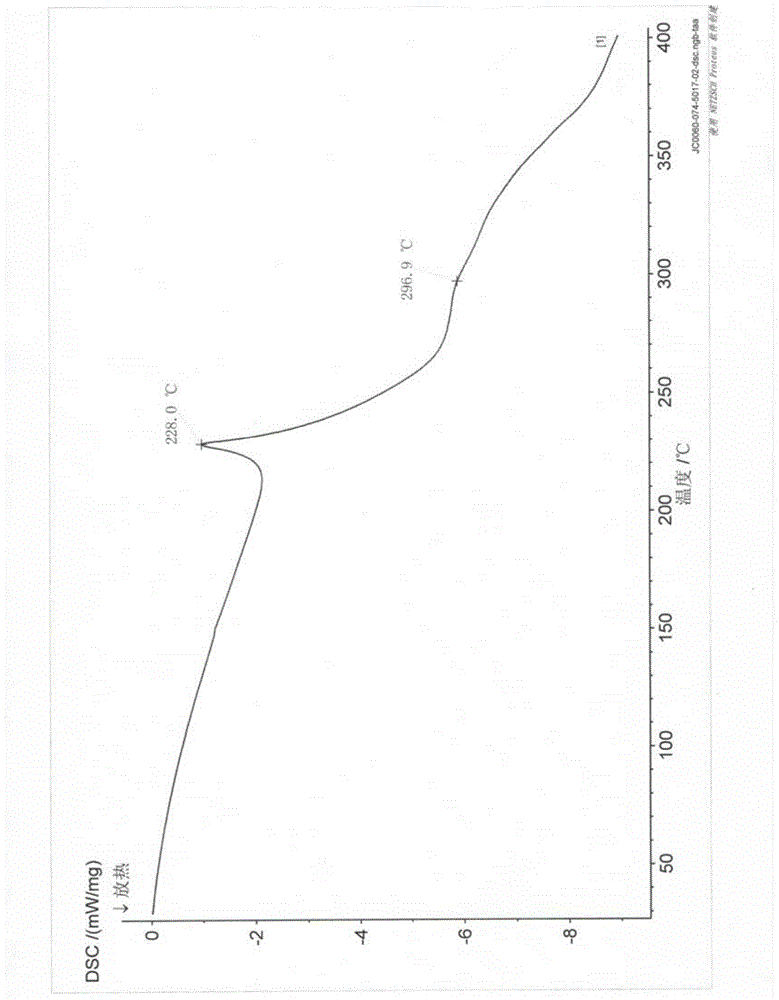
[0038] Carry out X-ray powder diffraction detection to present embodiment product, obtain as follows figure 1 and as figure 2 , carry out DSC detection to the present embodiment product, and as image 3 .
Embodiment 2
[0040] Preparation method of new crystal form of erlotinib hydrochloride
[0041] Add 5.0g of erlotinib base into a dry and clean 250mL four-necked bottle, add 75mL of methanol, heat to 40°C, stir for 30min, after the solid dissolves, heat filter to remove the insoluble matter, and pass in dry HCl under slow stirring Gas, stirred for 1h, cooled to 3±3°C under stirring, filtered for 1h, rinsed with 5mL of methanol, and the filter cake was dried in a blast oven at 30°C for 6h to obtain a new crystal form of erlotinib hydrochloride.
[0042] The quality of the product is 5.41 g, the yield is 99%, and the HPLC purity is 99.7%.
Embodiment 3
[0044] Preparation method of new crystal form of erlotinib hydrochloride
[0045] Add 5.0g of erlotinib base into a dry and clean 250mL four-necked bottle, add 75mL of isopropanol, heat to 50°C, and stir for 30min. HCl gas, stirred for 1.5h, cooled to room temperature under stirring, continued to cool to 7±3°C, continued to stir for 2h, filtered, rinsed with 5mL isopropanol, and dried the filter cake in a blast oven at 50°C for 8h to obtain A new crystal form of erlotinib hydrochloride.
[0046] The quality of the product is 5.41 g, the yield is 99%, and the HPLC purity is 99.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com