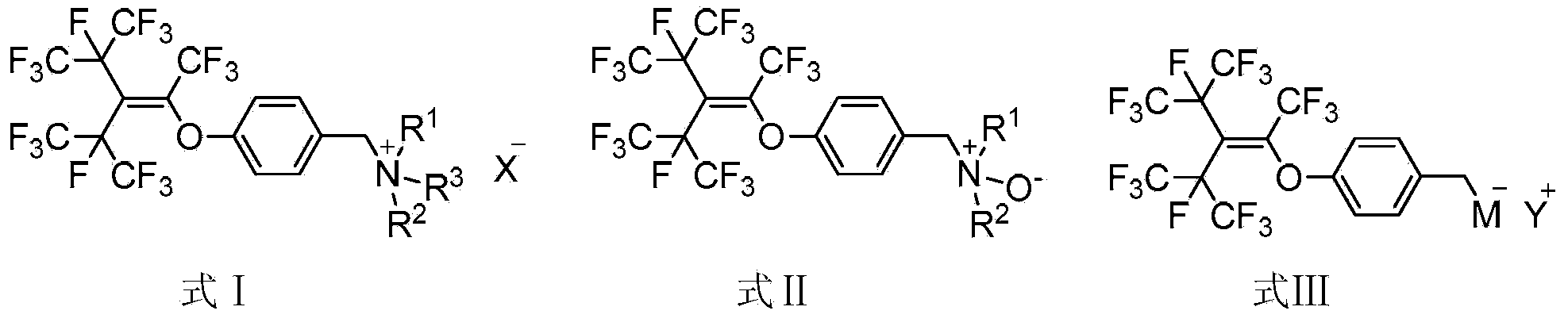
Surface active agent containing hexafluoropropylene tripolymer group and preparation method thereof
A technology of surfactant and hexafluoropropylene, which is applied in the preparation of organic compounds, amino hydroxyl compounds, sulfate esters, etc., can solve the problem of poor surface activity and poor stability of hexafluoropropylene trimer derivatives, which cannot meet the requirements of the application. Requirements and other issues to achieve good surface activity and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
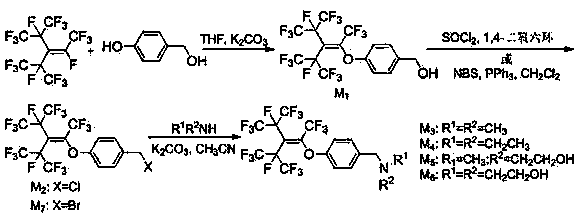
[0035] Embodiment 1 intermediate (M 1 ~ M 7 ) preparation
[0036] Intermediate (M 1 ~ M 7 ) synthetic route map as figure 1 shown.
[0037] Intermediate M 1 preparation of
[0038] Add 12.4g (0.1mol) p-hydroxybenzyl alcohol, 17.9g (0.13mol) potassium carbonate, 200mL THF (tetrahydrofuran) into a 1000mL three-necked flask, heat to reflux, and slowly add 58.5g (0.13mol) hexafluoropropylene trimer dropwise , continue to react for 2.5h, and TCL monitors the end of the reaction. Add 150mL ethyl acetate to the reaction solution, wash 3 times with saturated NaCl solution, and the organic layer is dried and precipitated to obtain the yellow liquid intermediate 4-perfluoro(4-methyl-3-isopropyl-2-pentane En-2-yl)oxybenzyl alcohol (M 1 ), 47.60g, and the yield was 86%. 1 H NMR (400MHz, CDCl 3 ):δ2.50(s,1H,-OH),4.61(s,2H,-CH 2 -),6.89(d,2H,J=7.8Hz,PhH),7.33(d,2H,J=7.8Hz,PhH); 19 F NMR (376MHz, CDCl 3 ):δ-56.93(d,3F,J=64.8Hz),-72.12(s,6F),-72.27(d,6F,J=38.8Hz),-168.27(d,6F,J...
Embodiment 2
[0055] The preparation of the surfactant containing hexafluoropropylene trimer base of embodiment 2 cationic series
[0056] N,N,N-Trimethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzyl ammonium iodide (A 1 ) preparation
[0057] Add 8.7 g (0.015 mol) of N,N-dimethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzylamine to a dry 250 mL flask (M 3 ), 4.3 g (0.03 mol) CH 3 I, 150 mL anhydrous CH 2 Cl 2 , react overnight at room temperature, filter with suction, and wash three times with ether to obtain a white solid as the target compound (A 1 ), 10.52g, the yield is 97%. 1 H NMR (400MHz,DMSO,δ,ppm):δ3.18(s,6H,N(CH 3 ) 2 ),δ4.44(s,2H,CH 2 ),δ6.96(d,2H,J=2.0Hz,PhH),δ7.62(d,2H,J=2.0Hz,PhH); 19 FNMR(376MHz,DMSO):δ-55.70(d,3F,J=60.9Hz),-70.92(s,6F),-71.99(s,6F),-167.31to-167.44(m,1F),-169.58 (s,1F);HPLC / MS(ESI):596.2(M + ) (calculated value: 596.09).
[0058]
[0059] N,N-Dimethyl-N-ethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzyl am...
Embodiment 3
[0092] Preparation of the hexafluoropropylene trimer-based surfactant of the amine oxide series of embodiment 3
[0093] N,N-Dimethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzylamine oxide (B 1 ) preparation
[0094] Add 6.4g (0.01mol) N,N-dimethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzylamine (M 3 ), 20mL30% (mass concentration) H 2 o 2 , 50mL methanol, reacted overnight at room temperature, desolvated, washed three times with ether to obtain a white solid as the target compound (B 1 ), 5.13g, the yield was 86%. 1 H NMR (600MHz, DMSO, δ, ppm): δ3.18(s, 6H, N-CH 3 ),δ4.44(s,2H,Ph-CH 2 -),δ6.96(d,2H,J=6.0Hz,PhH),δ7.62(d,2H,J=6.0Hz,PhH); 19 FNMR(376MHz,DMSO):δ-56.06(d,3F,J=36.5Hz),-70.73(d,6F),-72.90(s,6F),-167.51(s,1F),--169.71to- 170.78(m,1F);HPLC / MS(ESI):596.4(M + ) (calculated value: 597.06).
[0095]
[0096] N,N-diethyl-4-perfluoro(4-methyl-3-isopropyl-2-penten-2-yl)oxybenzylamine oxide (B 2 ) preparation
[0097] Add 7.3g (0.01m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com