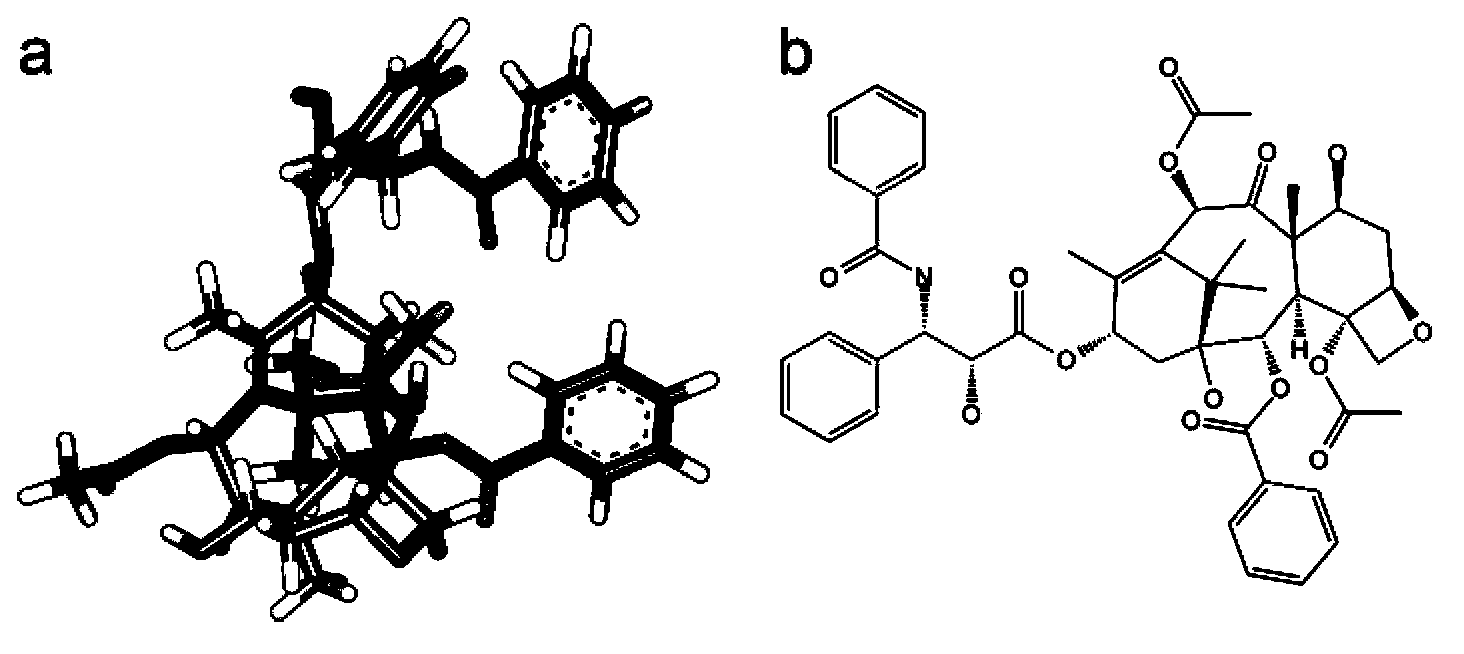
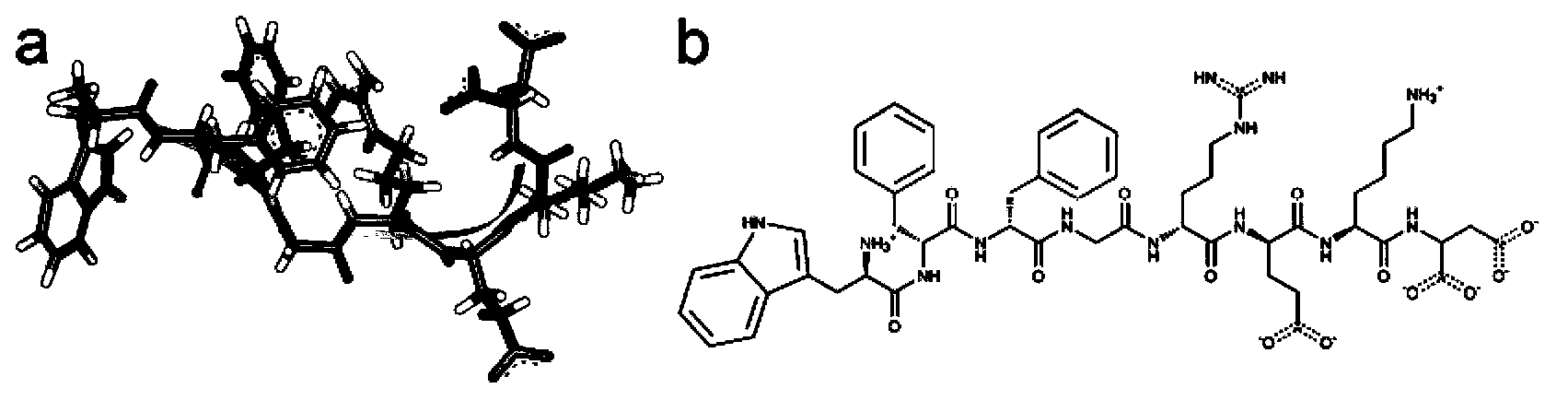
Oligopeptide used for increasing solubilities of paclitaxel or similar medicines based on paclitaxel structure
A paclitaxel and solubility technology, applied in the direction of drug combinations, peptides, anti-tumor drugs, etc., can solve the problem of low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of oligopeptide
[0058] The amino acid monomer [Trp(L), Trp(D), Phe(L), Phe(L), Ala(L), Ser(L), Gly(L), Arg(L), Glu, Lys(L) ), Asp(L)] are properly combined and connected to form various sequences.
[0059] Under the Amber software, the temperature of the above-mentioned various sequences was gradually raised in a recessive water environment with a step size of 50K, and then the molecular dynamics was stabilized at 300K for 250ns, and finally a stable oligopeptide conformation was obtained; using the docking software Auto Dock Vina docks the optimized oligopeptide molecule onto the paclitaxel molecule. Obtain a rough complex conformation and estimated binding free energy. For the AutoDock Vina program, the search space was limited to the center of the paclitaxel molecule, In the region, 10 optimal conformations were generated each time, and a total of 5 parallel operations were performed, and the conformation with the lowest binding fr...
Embodiment 2
[0066] Example 2: Solubilization preparation and determination of oligopeptide U1
[0067] Solubilization preparation of oligopeptide U1:
[0068]
[0069] Preparation and assay steps:
[0070] 1) Use absolute ethanol with 80% ethanol aqueous solution.
[0071] 2) Weigh 15mg of paclitaxel, dissolve it with ethanol aqueous solution and dilute to 1.5mg·mL -1 solution.
[0072] 3) Weigh 25mg U1, dissolve it in water and dilute to get 2.5mg·mL -1 solution.
[0073] 4) According to the volume ratio of 1:1, measure the two solutions into conical flasks with stoppers, place the conical flasks on a magnetic stirrer, 60°C, 300r·min -1 , stirred for 4 hours.
[0074] 5) Transfer the liquid in the Erlenmeyer flask to a round-bottomed flask, vacuum rotary evaporation, remove the polar organic solvent, transfer the residue to a 5mL volumetric flask with water several times, and make to volume.
[0075] 6) After the above solution was passed through a 0.22 μm filter membrane, 20 μL ...
Embodiment 3
[0077] Example 3: Solubilization preparation and determination of oligopeptide U2
[0078] Solubilization preparation of oligopeptide U2:
[0079]
[0080]
[0081] Preparation and assay steps:
[0082] 1) Use absolute ethanol with 80% ethanol aqueous solution.
[0083] 2) Weigh 15mg of paclitaxel, dissolve it with ethanol aqueous solution and dilute to 1.5mg·mL -1 solution.
[0084] 3) Weigh 25mg U2, dissolve it in water and dilute to get 2.5mg·mL -1 solution.
[0085] 4) According to the volume ratio of 1:1, measure the two solutions into conical flasks with stoppers, place the conical flasks on a magnetic stirrer, 60°C, 300r·min -1 , stirred for 4 hours.
[0086] 5) Transfer the liquid in the Erlenmeyer flask to a round-bottomed flask, vacuum rotary evaporation, remove the polar organic solvent, transfer the residue to a 5mL volumetric flask with water several times, and make to volume.
[0087] 6) After the above solution was passed through a 0.22 μm filter me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com