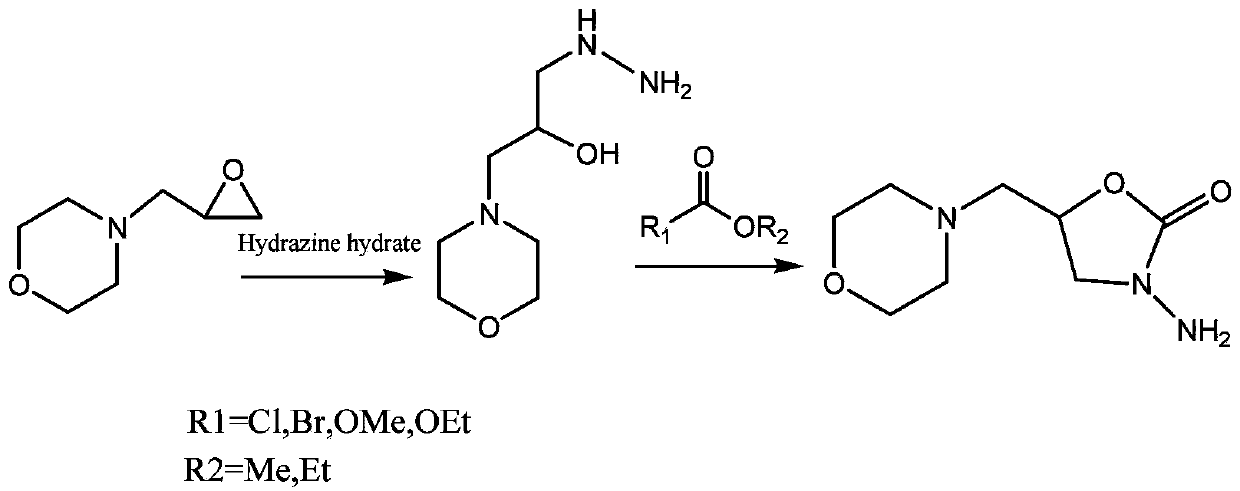
Synthesis method of 5- methylmorpholine-3-amino-2-oxazolidinyl ketone
A technology of oxazolidinone and methyl morpholine is applied in the field of veterinary drug synthesis and achieves the effects of high yield, good purity and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0025] Implementation example 15-methylmorpholine-3-amino-2-oxazolidinone preparation
[0026] (1) Add 14.3g morpholino propylene oxide and 20g80% hydrazine hydrate to a 100mL round bottom flask, stir and react at 60°C for 4 hours, then distill under reduced pressure (according to conventional methods) to remove water and excess hydrazine hydrate Hydrazine, in 1-hydrazino-3-morpholine-2-propanol.
[0027] (2) Dissolve 17.5g of 1-hydrazino-3-morpholine-2-propanol in 100ml of anhydrous methanol, add 20ml of dimethyl carbonate, add 8.0g of sodium methylate half an hour later, heat to reflux for 4 hours, After the reaction was completed, methanol was distilled off under reduced pressure (according to a conventional method), and 50 mL of ethanol was added to dissolve and then freeze at -20° C. to separate out 12 g of crystalline 5-methylmorpholine-3-amino-2-oxazolidinone (yield 60%,), the melting point is 116.3°C-118.5°C (the existing literature is 115-120°C).
Embodiment 25
[0028] Preparation of Example 25-methylmorpholine-3-amino-2-oxazolidinone
[0029] (1) Add 14.3g morpholino propylene oxide and 15g80% hydrazine hydrate to a 100mL round bottom flask, stir and react at 60°C for 6 hours, then distill under reduced pressure (according to conventional methods) to remove water and excess hydrazine hydrate Hydrazine to get 1-hydrazino-3-morpholine-2-propanol;
[0030] (2) Dissolve 17.5g of 1-hydrazino-3-morpholine-2-propanol in 100ml of absolute ethanol, add 20ml of ethyl chloroformate, add 8.0g of sodium ethoxide half an hour later, heat to reflux for 2 hours, After the reaction was completed, the ethanol was evaporated under reduced pressure (according to a conventional method), and 50 mL of ethanol was added to dissolve and then freeze at -20° C. to separate out 10 g of crystalline 5-methylmorpholine-3-amino-2-oxazolidinone (yield 50%), melting point 115.5°C-118.3°C (existing literature is 115-120°C).
Embodiment 35
[0031] Preparation of Example 35-methylmorpholine-3-amino-2-oxazolidinone
[0032] (1) Add 29g morpholino propylene oxide and 40g80% hydrazine hydrate to a 100mL round bottom flask, stir and react at 60°C for 8 hours, then distill under reduced pressure (according to conventional methods) to remove water and excess hydrazine hydrate , in 1-hydrazino-3-morpholine-2-propanol.
[0033] (2) Dissolve 35g of 1-hydrazino-3-morpholine-2-propanol in 200ml of isopropanol, add 40ml of ethyl chloroformate, add 15.0g of sodium ethylate half an hour later, heat to reflux for 8 hours, and react At the end, the isopropanol was distilled off under reduced pressure (according to the conventional method), and 80 mL of ethanol was added to dissolve it and freeze at -20°C to precipitate 13 g of crystalline 5-methylmorpholine-3-amino-2-oxazolidinone (produced rate 65%), melting point 117.5°C-120.3°C (existing literature is 115-120°C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com