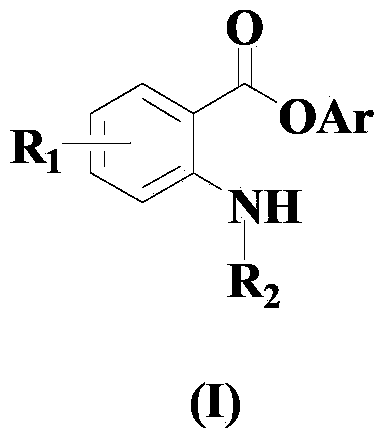
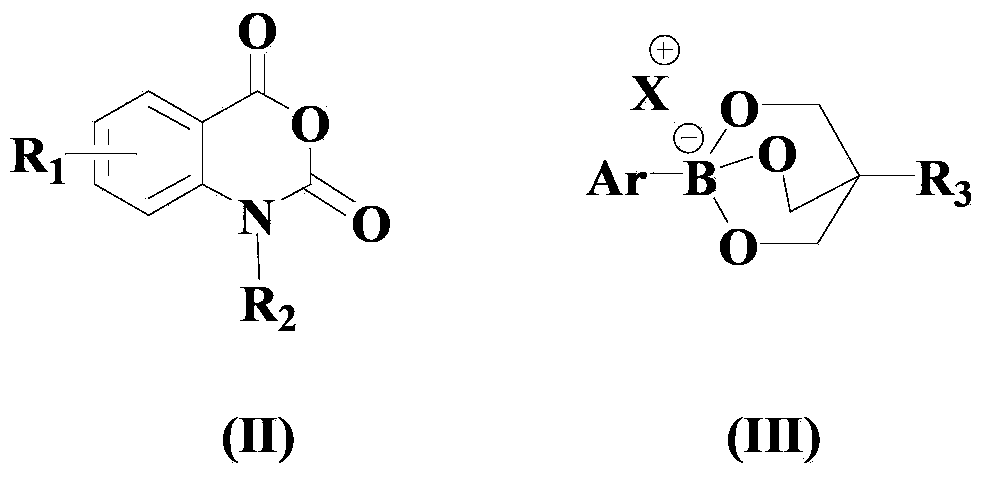
Method for compounding amino-substituted arylate compound
A synthesis method and compound technology, which are applied in the synthesis of aryl ester compounds and the synthesis of amino-substituted aryl ester compounds, can solve the problems of low yield, selectivity still needs to be further improved, and limit the possibility of larger-scale application. Achieve the effect of avoiding the use of precious metals and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of phenyl anthranilate
[0061]
[0062] In a dry and clean reactor, add 50ml solvent THF, then add the above formula (II) compound, formula (III) compound, formula (a) copper compound and 1,10-phenanthroline in sequence, so that the molar ratio is 1 :1:0.05:0.1, wherein the compound of formula (II) is 10mmol, and the reaction system is stirred and reacted at 40°C for 15 hours.
[0063] After the reaction was finished, filter, and the filtrate was rotary evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 200-300 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 99.4% and a purity of 99.1% ( HPLC).
[0064] Melting point: 70-71°C;
[0065] NMR: 1 H NMR (500MHz, CDCl 3 )δ5.76(s,2H),6.67-6.72(m,2H),7.14-7.20(m,2H),7.27-7.29(m,1H),7.30-7.35(m,1H),7.39-7.45( tm,2H), 8.07-8.10(m,1H).
Embodiment 2
[0066] Embodiment 2: the synthesis of m-methylphenyl anthranilic acid
[0067]
[0068] In a dry and clean reactor, add 50ml of solvent acetone, then add the above formula (II) compound, formula (III) compound, formula (b) copper compound and 1,10-phenanthroline monohydrate successively to make the mole The ratio is 1:1.5:0.1:0.2, wherein the compound of formula (II) is 10 mmol, and the reaction system is stirred and reacted at 50° C. for 20 hours.
[0069] After the reaction was completed, filter, and the filtrate was evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product with a yield of 97.6% and a purity of 98.2% (HPLC).
[0070] NMR: 1 H NMR (500MHz, CDCl 3 )δ2.41(s,3H),5.76(s,2H),6.70-6.75(m,2H),6.98-7.03(m,2H),7.07-7.11(m,1H),7.31-7.37(m, 2H), 8.09-8.11 (m, 1H).
Embodiment 3
[0071] Embodiment 3: the synthesis of p-methoxyphenyl anthranilic acid
[0072]
[0073] In a dry and clean reactor, add 50ml of solvent HMPA, then add the above formula (II) compound, formula (III) compound, formula (a) copper compound and 1,10-phenanthroline in turn, so that the molar ratio is 1 :2:0.15:0.3, wherein the compound of formula (II) is 10mmol, and the reaction system is stirred and reacted at 60°C for 25 hours.
[0074] After the reaction was finished, filter, and the filtrate was rotary evaporated with a rotary evaporator to remove the solvent, and the residue was purified by 400-500 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 76.7% and a purity of 97.9% ( HPLC).
[0075] Melting point: 102-103°C;
[0076] NMR: 1 H NMR (500MHz, CDCl 3 )δ3.85(s,3H),5.78(s,2H),6.72(d,J=7.8Hz,2H),6.94(d,J=7.2Hz,2H),7.08-7.12(m,2H), 7.30-7.36 (m, 1H), 8.09-8.11 (m, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com