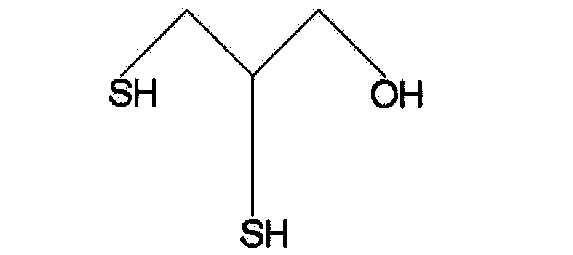
Method for resolution of 2,3-dimercapto propanol raceme
A dimercaptopropanol, racemate technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effect of precise medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1) Under nitrogen protection, add 10g (80mmol) of dimercaptopropanol racemate to 40ml (S)-(+)-2-butanol, and add 7g (80mmol) (R)-(- )-3-methyl-2-butylamine, after the dropwise addition, continue to stir the reaction for 3h; 40mmol) of (S)-(+)-2-butanol solution, after the dropwise addition, continue to stir and react for 0.5h, let stand, and filter;
[0070] 2) Add the filter cake obtained in step 1) into 15ml of toluene, under stirring, add 40ml of toluene solution containing 80mmol hydrogen chloride, filter, concentrate, recover toluene, and obtain (R)-(-)-2,3-dimercapto Propanol 4.3g, [α]=-3.77°, the purity of 2,3-dimercaptopropanol is 98.95%;
[0071] 3) Concentrate the filtrate obtained in step 1) to recover (S)-(+)-2-butanol, add 15ml of toluene, under stirring, add 50ml of toluene solution containing 100mmol hydrogen chloride, filter, concentrate, and recover toluene to obtain (S )-(+)-2,3-dimercaptopropanol 5.0g, [α]=+3.15°, the purity of 2,3-dimercaptopropano...
Embodiment 2
[0073] 1) Under nitrogen protection, add 10g (80mmol) of dimercaptopropanol racemate to 50ml (S)-(+)-2-butanol, and drop 7g (80mmol) of (R)-(- )-3-methyl-2-butylamine, after the dropwise addition, continue to stir the reaction for 3h; 40mmol) of (S)-(+)-2-butanol solution, after the dropwise addition, continue to stir and react for 0.5h, let stand, and filter.
[0074] 2) Concentrate the filtrate obtained in step 1), add 15ml of toluene to the obtained product, add 50ml of toluene dilution solution containing 100mmol hydrogen chloride under stirring, filter, concentrate, and recover toluene to obtain (S)-(+)-2, 5.1 g of 3-dimercaptopropanol, [α]=+2.85°, the purity of 2,3-dimercaptopropanol is 98.71%.
[0075] 3) Add the filter cake obtained in step 1) into 15ml of toluene, under stirring, add 80ml of toluene solution containing 80mmol hydrogen chloride, filter, concentrate, and recover toluene to obtain (R)-(-)-2,3-dimercapto 3.9 g of propanol, [α]=-3.92°, and the purity of ...
Embodiment 3
[0077]1) Under nitrogen protection, add 10g (80mmol) of dimercaptopropanol racemate to 30ml (S)-(+)-2-butanol, and drop 7g (80mmol) of (R)-(- )-3-methyl-2-butylamine, after the dropwise addition, continue to stir the reaction for 3h; 80mmol) of (S)-(+)-2-butanol solution, after the dropwise addition, continue to stir and react for 0.5h, let stand, and filter.
[0078] 2) Add the filter cake obtained in step 1) into 15ml of toluene, under stirring, add 40ml of toluene solution containing 80mmol hydrogen chloride, filter, concentrate, recover toluene, and obtain (R)-(-)-2,3-dimercapto Propanol 4.5g, [α]=-3.65°, the purity of 2,3-dimercaptopropanol is 99.03%;
[0079] 3) Concentrate the filtrate obtained in step 1) to recover (S)-(+)-2-butanol, add 15ml of toluene, under stirring, add 50ml of toluene solution containing 100mmol hydrogen chloride, filter, concentrate, and recover toluene to obtain (S )-(+)-2,3-dimercaptopropanol 4.9g, [α]=+3.37°, the purity of 2,3-dimercaptoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com