A kind of synthetic method of β-amino-alpha-hydroxycyclobutanamide hydrochloride
A technology of hydroxycyclobutylbutanamide and synthetic method, which is applied in the field of synthesis of boceprevir intermediates, can solve the problems of low product purity and yield, unfavorable industrial production, unfavorable continuous production, etc., and achieve improved yield and The effects of purity, reduced need for auxiliary raw materials, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
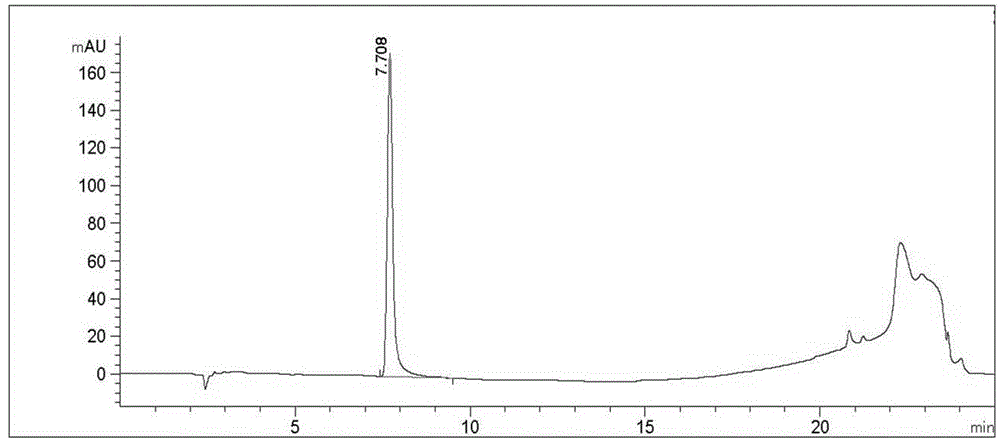
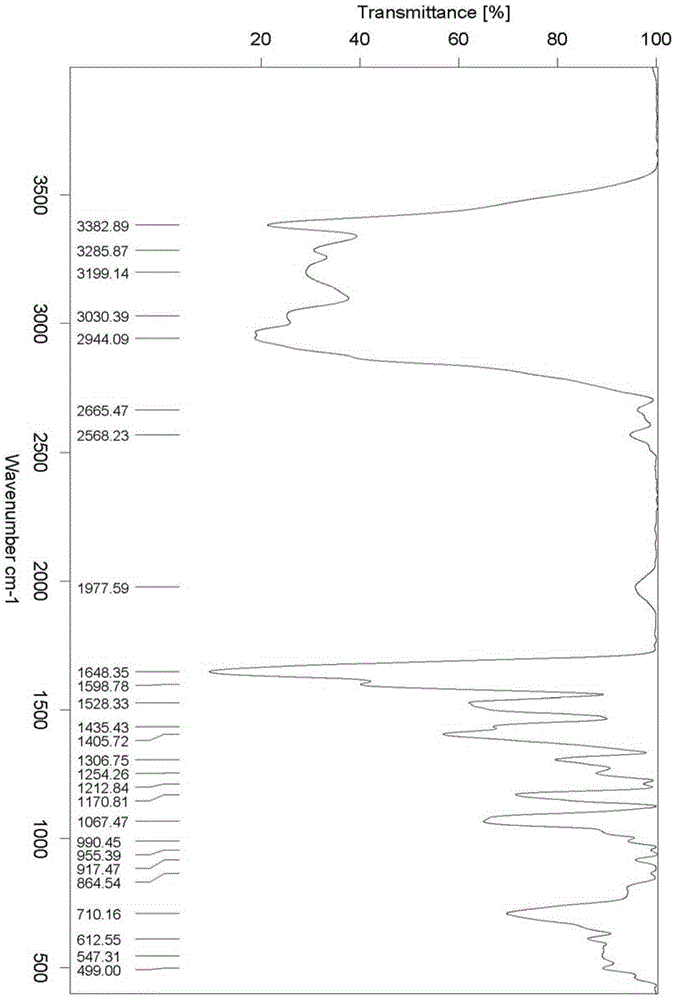
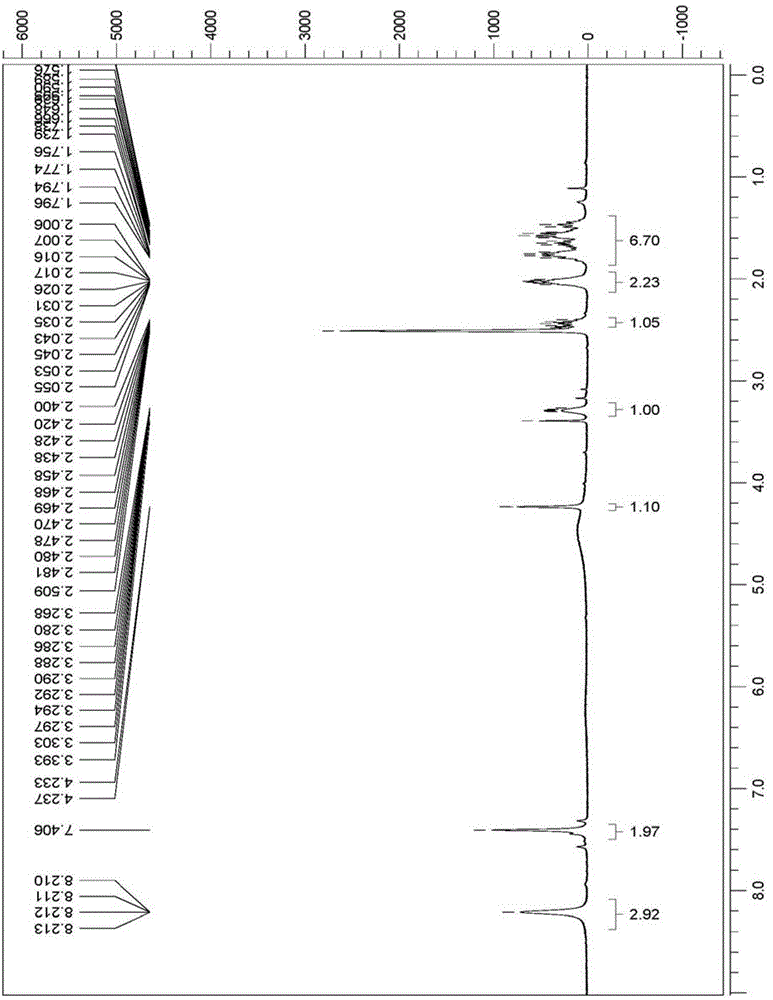
Image
Examples
Embodiment 1
[0039] Dissolve 150g Boc-tert-butyl glycine in 1.5L THF, stir for 0.5h, cool down to -30°C; slowly add 648ml LDA dropwise to the system, keep warm and continue stirring for 0.5h; add dropwise 96.7g cyclobutane to the system methyl bromide, drop the system and continue to keep warm at -30°C for 2 hours; add 1.5L of 10% ammonium chloride aqueous solution and 1.5L MBTE to the system, extract and separate the liquid, extract the aqueous phase with 1.5L MBTE, combine the organic phases, and use Wash with 1.5L saturated brine, then wash with 300g anhydrous NaSO 4 After drying and suction filtration, the filtrate was concentrated under reduced pressure to no solvent to obtain tert-butyl 2-tert-butoxycarbonylamino acid-3-cyclobutylpropionate with a yield of 90.0%.
[0040] Dissolve 150g of 2-tert-butoxycarbonyl amino acid-3-cyclobutylpropionate tert-butyl in 1.5LTHF, lower the temperature to -20°C under the protection of nitrogen, and slowly add 551mL of 1.1eq diisobutylaluminum hydri...
Embodiment 2
[0047] Dissolve 150g Boc-glycine tert-butyl ester in 2.0L methyl tert-butyl ether, stir for 20 minutes, cool down to -40°C; slowly add 1000ml HMDSLi dropwise to the system, keep warm and continue stirring for 20 minutes; drop into the system Add 136g of cyclobutylchloromethane, drop the system and continue to keep warm at -40°C for 2 hours; add 2.0L of 10% ammonium chloride aqueous solution and 2.0L of MBTE to the system, extract and separate the liquid, and then extract the water phase with 2.0L of MBTE. The organic phases were combined, washed with 2.0L saturated brine, and then washed with 450g of anhydrous NaSO 4 After drying and suction filtration, the filtrate was concentrated under reduced pressure to no solvent to obtain tert-butyl 2-tert-butoxycarbonylamino acid-3-cyclobutylpropionate with a yield of 91.6%.
[0048] Dissolve 150g of 2-tert-butoxycarbonyl amino acid-3-cyclobutylpropionate tert-butyl in 2.0L of methyl tert-butyl ether, lower the temperature to -20°C und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com