Method for preparing piribedil in high-purity high-yield manner
A high-yield technology for piribedil, which is applied in the new synthetic field of preparing piribedil, can solve the problems of low product yield and low synthetic route, and achieve high total yield, less side reactions, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
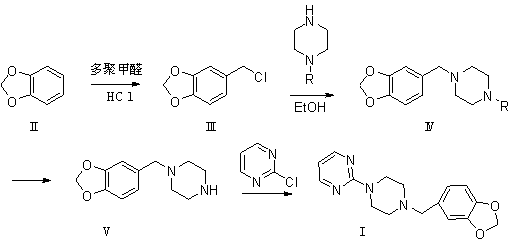
[0066] 4) Preparation of formula (I) piribedil
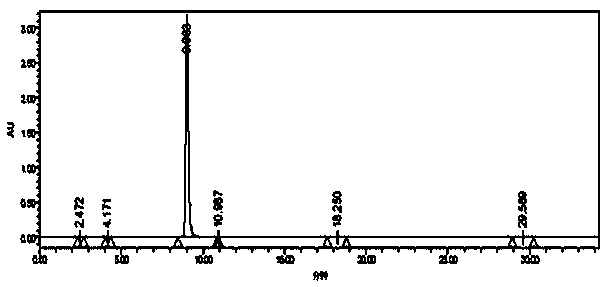
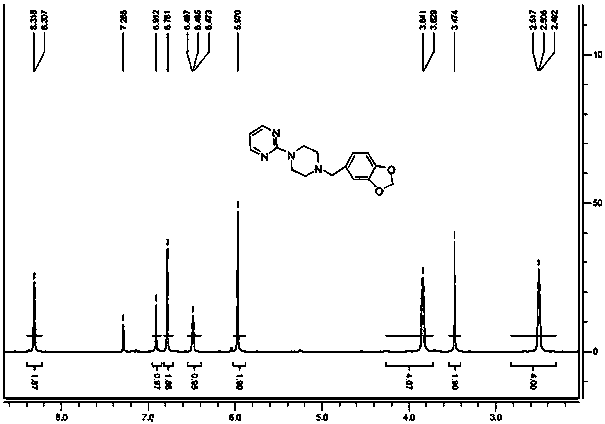
[0067] Dissolve 1-(3,4-methylenedioxybenzyl)piperazine of formula (V) in an appropriate solvent, add 2-chloropyrimidine, react at a suitable temperature under alkaline conditions, and the reaction is complete Afterwards, through appropriate aftertreatment, obtain product piribedil formula (I).
[0068]
[0069] The suitable solvent is at least any one of water, methanol, ethanol, isopropanol, acetonitrile, methylene chloride or chloroform, but is not limited thereto. In preferred embodiments of the present invention, it is preferred that the reaction be carried out in ethanol or carried out in methanol; the suitable temperature is -20°C to 100°C, and the preferred reaction temperature is 60°C to 80°C; the alkali in the alkaline condition is selected from sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, At least any one of triethylamine or ammonia, but not limited thereto, preferably potassium car...
Embodiment 1
[0071] Example 1 Preparation of 3,4-methylenedioxybenzyl chloride
[0072] Add 1.0kg (8.19mol) of piperonine, 3L of concentrated hydrochloric acid, 0.05kg of tetrabutylammonium bromide, 0.83kg (9.22mol) of paraformaldehyde into a 10L flask, stir at a low temperature of 0°C to 5°C for half an hour, then continue to heat up React at 15°C to 20°C for 2 hours. Add dichloromethane to extract 3kg*3 (extraction 3 times), wash the organic layer with water, add anhydrous sodium sulfate to dry for 6 hours, and concentrate to dryness to obtain a colorless and transparent oily product. The crude product is obtained by vacuum distillation to obtain 3, 4-Methylenedioxybenzyl chloride, 1.02kg (5.98mol), yield 74%.
Embodiment 2
[0073] Example 2 Preparation of 3,4-methylenedioxybenzyl chloride
[0074] Add 1kg (8.19mol) of piperonine, 3L of concentrated hydrochloric acid, 0.05kg of PEG-200, 0.73kg (8.1mol) of paraformaldehyde into a 10L flask, stir at a low temperature of -10°C to 0°C for half an hour, then continue to heat up to 20°C to React at 30°C for 2 hours. Add dichloromethane to extract 3kg*3, wash the organic layer with water, add anhydrous sodium sulfate to dry for 6 hours, and then concentrate to dryness to obtain a colorless and transparent oily product. After rectification, 3,4-methylenedioxybenzyl chloride is obtained. 1.08kg (6.33mol), yield 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com