High-specific-surface-area perovskite catalyst LaCo0.9Mg0.1O3 and preparation method thereof
A high specific surface area, perovskite technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as gaps, and achieve easy operation, process Simple, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
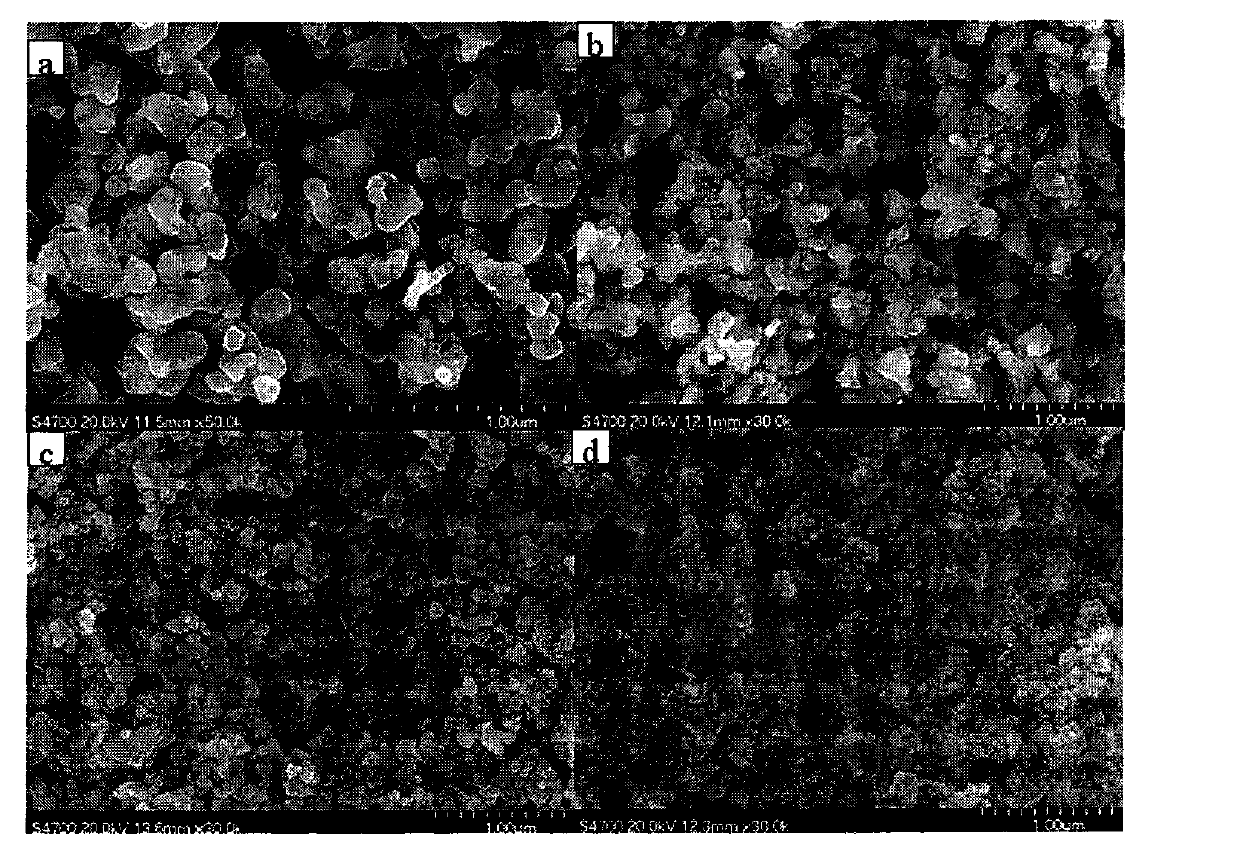
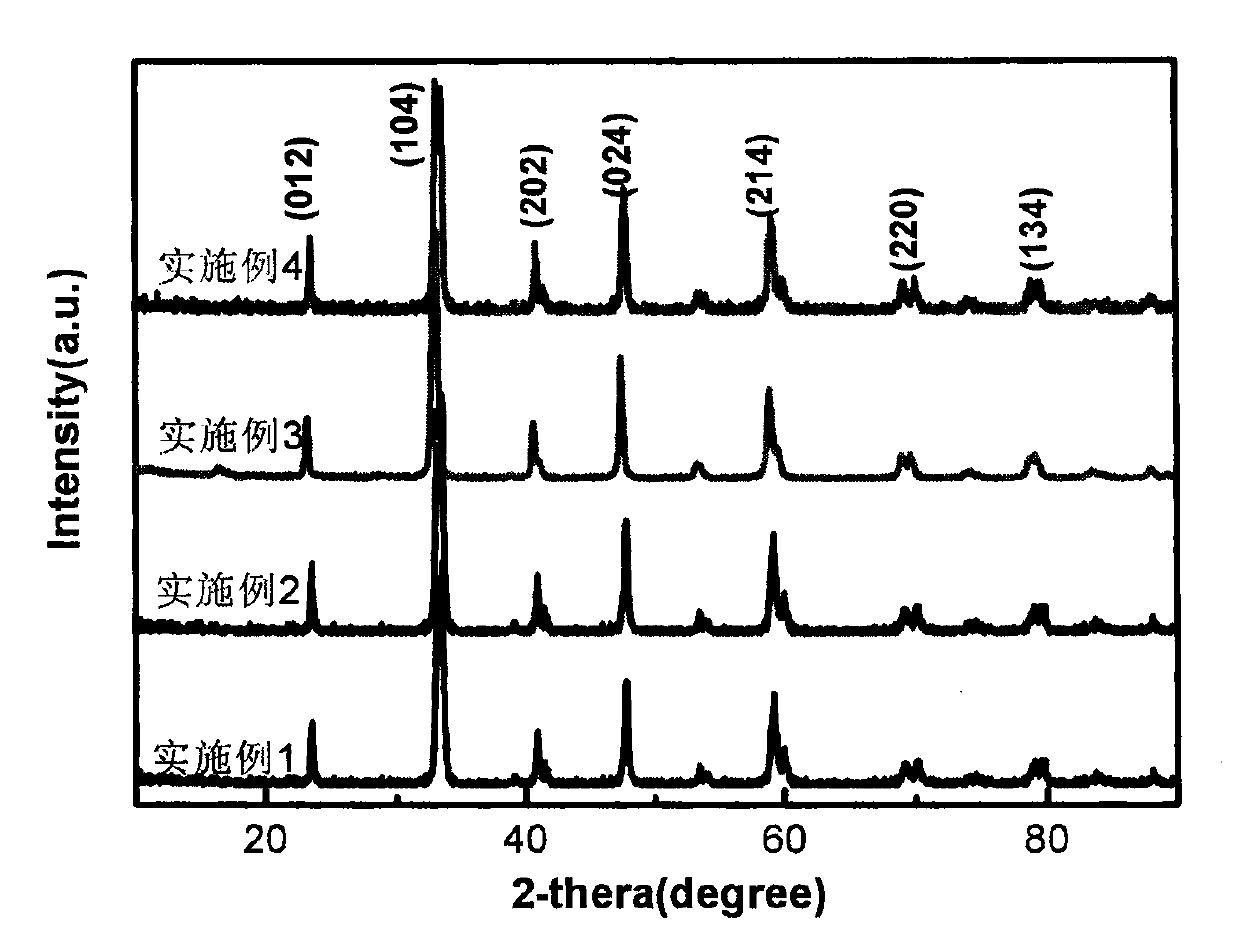
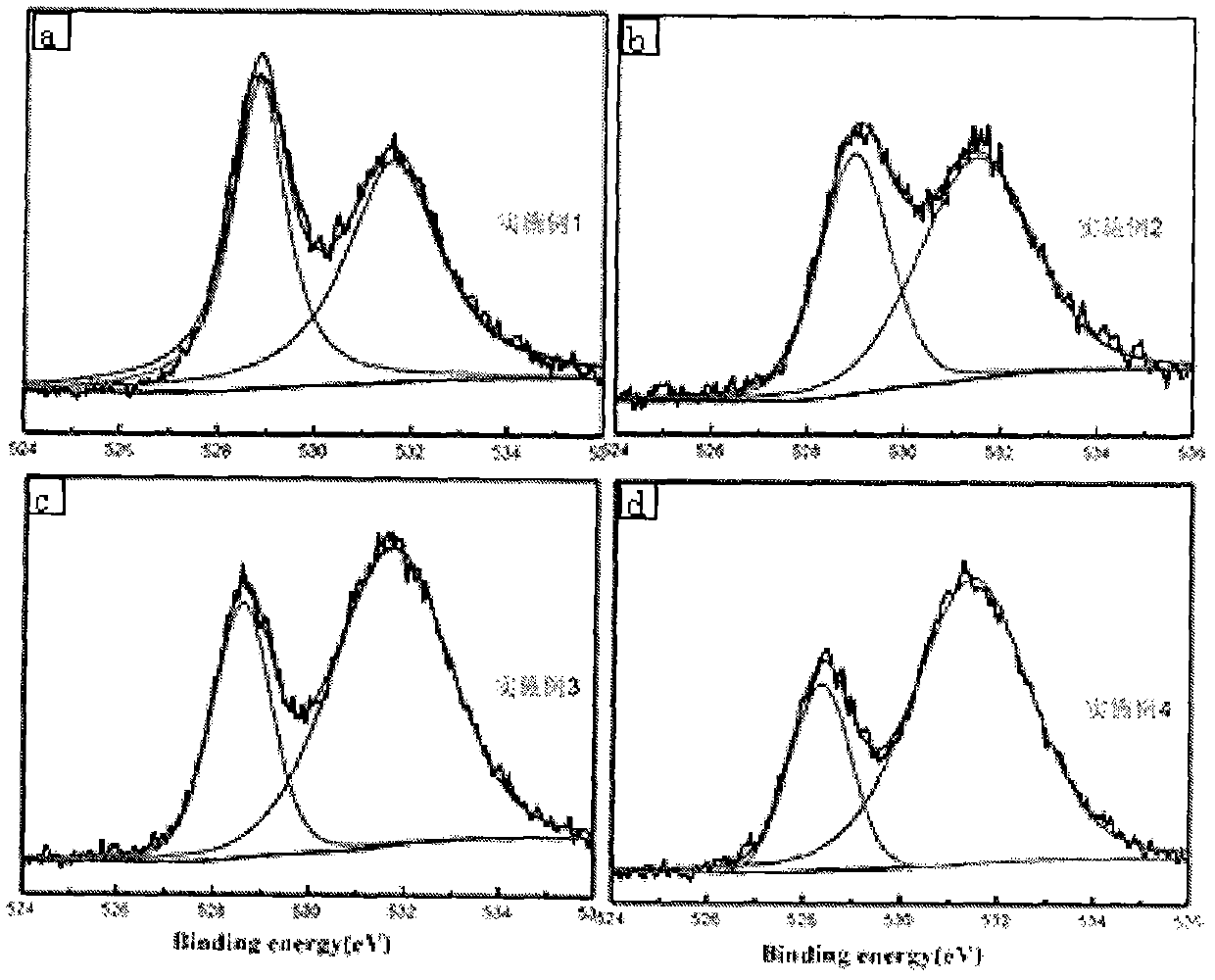
[0024] Embodiment 1: take by weighing 2.165g La(NO 3 ) 3 ·6H 2 O, 1.31g Co(NO 3 ) 3 ·6H 2 O and 0.128g Mg(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of deionized water, 0.2 glycine was added thereto, and after magnetic stirring for 1 h, a homogeneous solution was formed. Place the above solution in a water bath at 80°C, stir and evaporate to dryness until it reaches a sol state; place it in an oven at 105°C to dry to obtain a precursor, and firstly bake the precursor in a muffle furnace at a heating rate of 3°C / min to a temperature of 400°C 4h, and then the roasted precursor was raised to a temperature of 700°C in a muffle furnace at a heating rate of 3°C / min and roasted for 4h to obtain a perovskite powder; the obtained perovskite powder was added to a concentration of 0.4mol / In 1 L of acetic acid solution, magnetically stirred for 1 h to remove excess magnesia solids, washed the obtained sample with deionized water, centrifuged, and dried in an oven at 80°C for 12 h to...
Embodiment 2
[0029] Embodiment 2: take by weighing 2.165g La(NO 3 ) 3 ·6H 2 O, 1.31g Co(NO 3 ) 3 ·6H 2 O and 1.28g Mg(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of deionized water, 2 g of glycine was added thereto, and after magnetic stirring for 1 h, a homogeneous solution was formed. Place the above solution in a water bath at 80°C, stir and evaporate to dryness until it reaches a sol state; place it in an oven at 105°C to dry to obtain a precursor, and firstly bake the precursor in a muffle furnace at a heating rate of 3°C / min to a temperature of 400°C 4h, and then the roasted precursor was raised to a temperature of 700°C in a muffle furnace at a heating rate of 3°C / min and roasted for 4h to obtain a perovskite powder; the obtained perovskite powder was added to a concentration of 0.4mol / In 1 L of acetic acid solution, magnetically stirred for 1 h to remove excess magnesia solids, washed the obtained sample with deionized water, centrifuged, and dried in an oven at 80°C for 12 h ...
Embodiment 3
[0032] Embodiment 3: take by weighing 2.165g La(NO 3 )e 3 ·6H 2 O, 1.31g Co(NO 3 ) 3 ·6H 2 O and 2.56g Mg(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of deionized water, 1.46 g of glycine was added thereto, and after magnetic stirring for 1 h, a homogeneous solution was formed. Place the above solution in a water bath at 80°C, stir and evaporate to dryness until it reaches a sol state; place it in an oven at 105°C to dry to obtain a precursor, and firstly bake the precursor in a muffle furnace at a heating rate of 3°C / min to a temperature of 400°C 4h, and then the roasted precursor was raised to a temperature of 700°C in a muffle furnace at a heating rate of 3°C / min and roasted for 4h to obtain a perovskite powder; the obtained perovskite powder was added to a concentration of 0.4mol / In 1 L of acetic acid solution, magnetically stirred for 1 h to remove excess magnesia solids, washed the obtained sample with deionized water, centrifuged, and dried in an oven at 80°C for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com