Industrialization preparation method for 4-fluoroisatin and product thereby
A technology of fluoroisatin and products, which is applied in the field of preparation of heterocyclic compounds, and achieves the effects of low energy consumption, low cost, and convenient quality control and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
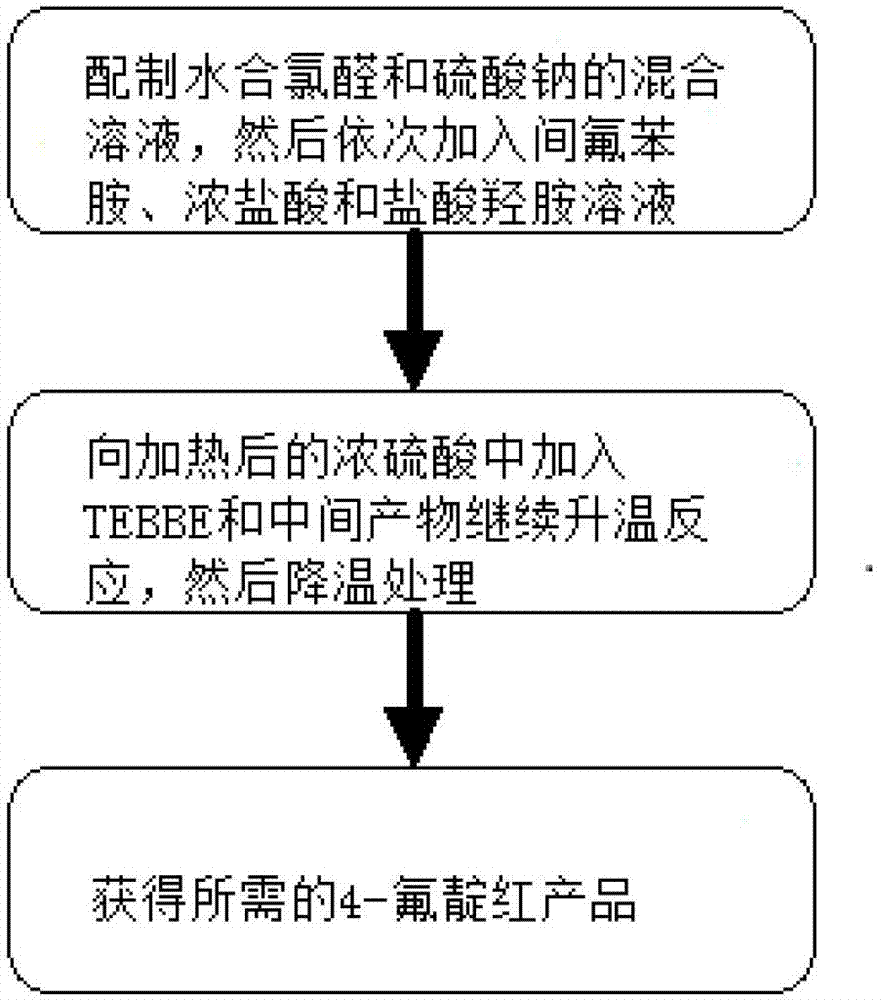
[0030] The first step: Oxime formation reaction
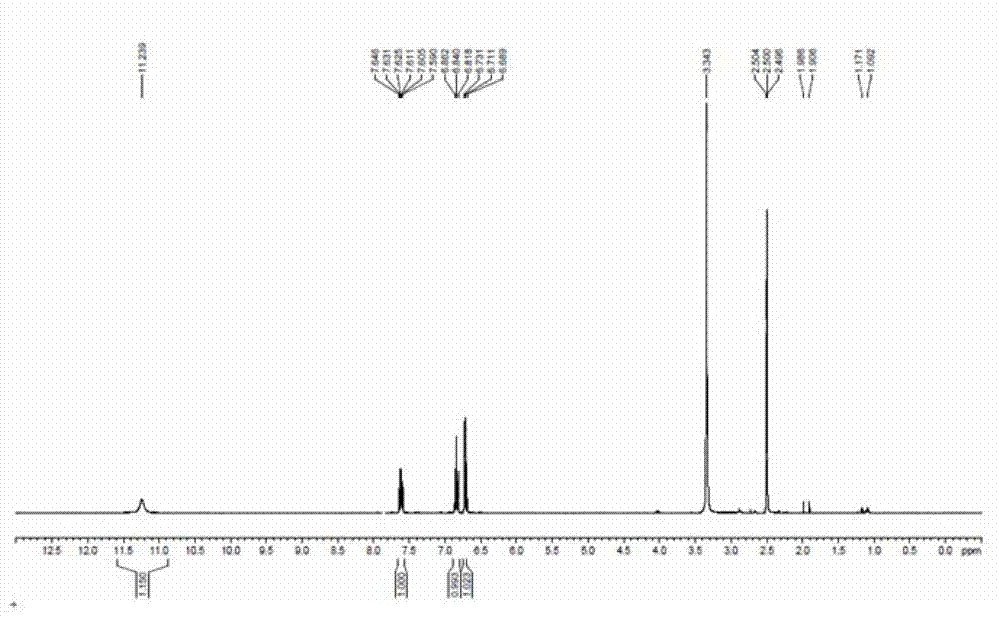
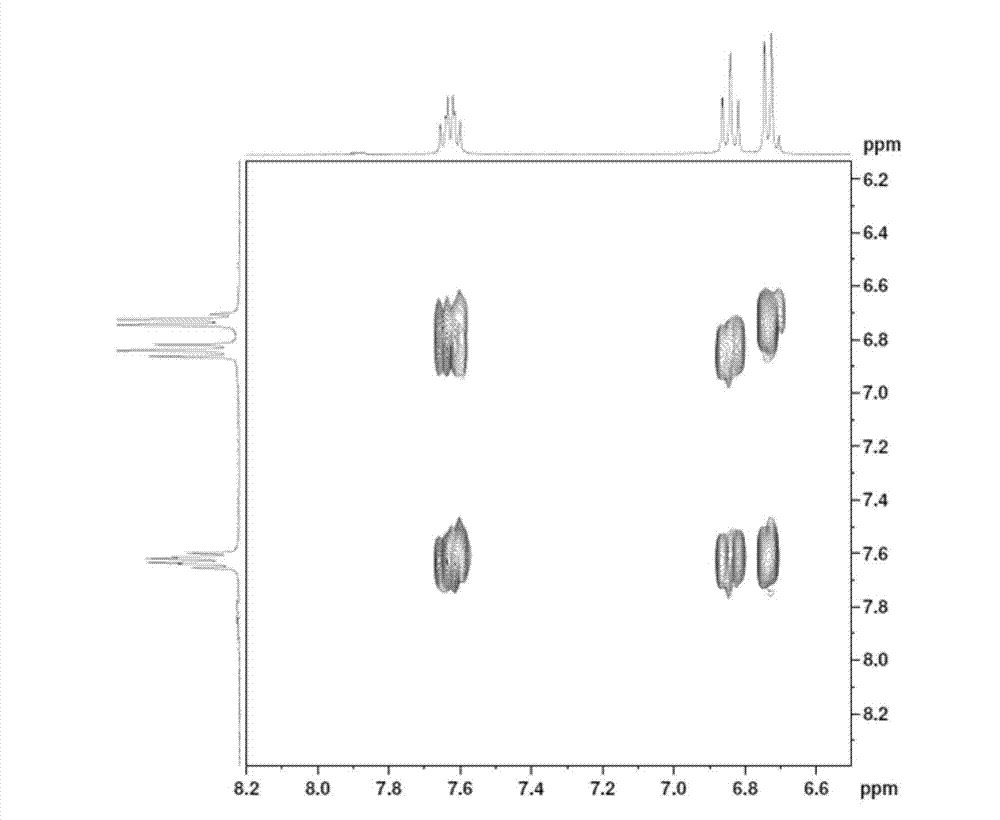
[0031] Chloral hydrate (95.7 grams, 0.58 moles) and sodium sulfate (448.5 grams, 3.15 moles) were dissolved in 2.2 liters of water, heated to 30-35 degrees, reacted for 15 minutes, and then added m-fluoroaniline (58.8 grams, 0.53 moles) , 57 ml concentrated hydrochloric acid, 200 ml aqueous solution of hydroxylamine hydrochloride (109.7 g, 1.58 mol). After the addition, heat to 80-90 degrees to react for 2 hours, then cool to 50 degrees to filter, wash the filter cake with water, and dry to obtain 86 grams of product (yield 89.5%).
[0032] Step 2: Ring Closing Reaction
[0033] Heat 430 milliliters of concentrated sulfuric acid to 50-60 degrees, add 8.6 grams of TEBBE reagent, add product 2 (86 g, 0.47 moles) in batches, the temperature cannot exceed 65 degrees, after the addition is completed, heat up to 80-85 degrees for 15 minutes, and then Cool down to room temperature, pour into 1.2 liters of ice water, an orange-red pr...
Embodiment 2
[0035] The first step: Oxime formation reaction
[0036] Chloral hydrate (287 grams, 1.74 moles) and sodium sulfate (1.34 kilograms, 9.45 moles) were dissolved in 6.6 liters of water, heated to 30-35 degrees, reacted for 15 minutes, and then added m-fluoroaniline (176.4 grams, 1.59 moles) successively, For example, 171 milliliters of concentrated hydrochloric acid with a mass fraction of more than 37%, 600 milliliters of an aqueous solution of hydroxylamine hydrochloride (329.4 grams, 4.74 moles). After the addition, heat to 80-90 degrees to react for 2 hours, then cool down to 50 degrees to filter, wash the filter cake with water, and dry to obtain 272 grams of product (yield 93.9%).
[0037] Step 2: Ring Closing Reaction
[0038] Heat 1.4 liters of concentrated sulfuric acid with a mass percentage concentration of, for example, 85% or more to 50-60 degrees, add 27 grams of TEBBE reagent, and add product 2 (272 g, 1.5 moles) in batches. The temperature cannot exceed 65 degre...
Embodiment 3
[0040] The first step: Oxime formation reaction
[0041] Chloral hydrate (2.8 kg, 17.4 moles) and sodium sulfate (18 kg, 127.2 moles) were dissolved in 66 liters of water, heated to 30-35 degrees, reacted for 15 minutes, and then added m-fluoroaniline (1.76 kg, 15.9 moles) , 1.91 liters of concentrated hydrochloric acid, 6 liters of aqueous solution of hydroxylamine hydrochloride (3.3 kg, 47.4 moles). After the feeding is completed, heat to 80-90 degrees to react for 2 hours, then cool down to 50 degrees to filter, wash the filter cake with water, and dry to obtain 2.84 kg of product (yield 98.1%).
[0042] Step 2: Ring Closing Reaction
[0043] Heat 14 liters of concentrated sulfuric acid to 50-60 degrees, add 142 grams of TEBBE reagent in batches, add product 2 (2.84 kg, 15.6 moles) in batches, the temperature cannot exceed 65 degrees, and heat up to 80 degrees for 15 minutes after the addition. Cool down to room temperature, pour into ice water, an orange-red precipitate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com