Compound amino acid composition and preparation method thereof
A technology of compound amino acids and compositions, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as unsuitable for popularization and application and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Prepare 5 L of saturated aqueous solution of crude L-tryptophan at 25°C;
[0059] (2) In a sound field with a frequency of 25KHz and an output power of 40W, under the condition of nitrogen filling, add a mixed solution of isopropanol and ether at 5°C while stirring, stop the sound field after adding the mixed solution, and cool down while stirring. When the temperature was lowered to 0°C, the stirring was stopped, and the crystal was grown at 0°C for 6 hours. After the crystals were precipitated, they were washed and dried to obtain the L-tryptophan compound. Wherein, the volume of the mixed solution of isopropanol and ether added is 25L; the volume ratio of isopropanol and ether is 3:2; the adding speed of the mixed solution of added isopropanol and ether is: v 1 =250ml / min; the speed of cooling is v 2 =5°C / hour.
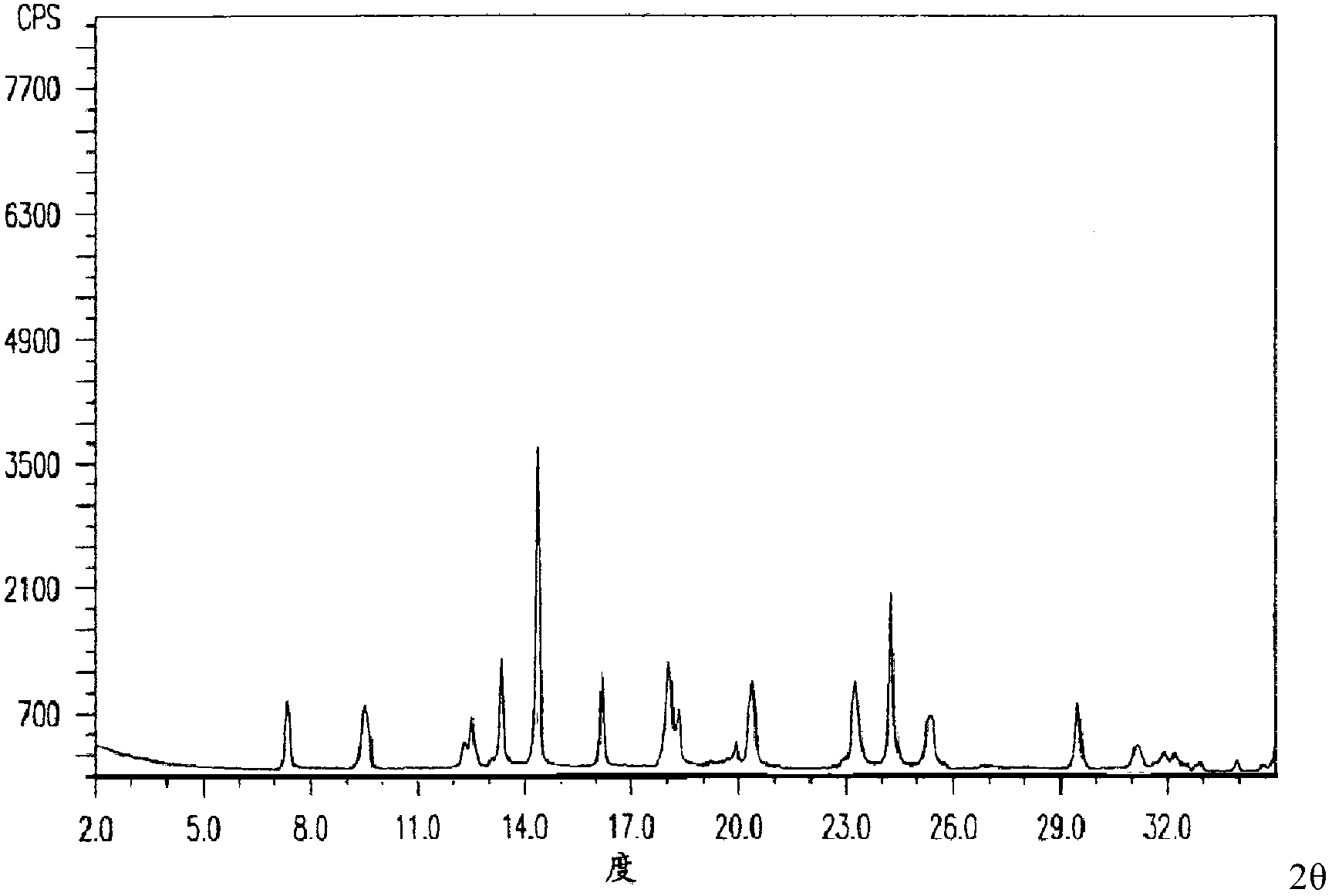
[0060] The X-ray powder diffraction pattern obtained by measuring the L-tryptophan compound using Cu-Kα rays is as follows: figure 1 As shown, the pu...
Embodiment 2
[0062] (1) Prepare 10L of a saturated aqueous solution of crude L-tryptophan at 30°C;
[0063] (2) In a sound field with a frequency of 25KHz and an output power of 60W, under the condition of nitrogen filling, add a mixed solution of isopropanol and ether at 0°C while stirring, stop the sound field after adding the mixed solution, and cool down while stirring. When the temperature was lowered to 0°C, the stirring was stopped, and the crystal was grown at 0°C for 2 hours. After the crystals were precipitated, the crystals were washed and dried to obtain the L-tryptophan compound. Wherein, the volume of the mixed solution of isopropanol and ether added is 30L of the saturated aqueous solution of L-tryptophan crude product; the volume ratio of isopropanol and ether is 3:1; the adding speed of the mixed solution of added isopropanol and ether is :v 1 =300ml / min; the speed of cooling is v 2 =6°C / hour.
[0064] The X-ray powder diffraction pattern obtained by measuring the L-try...
Embodiment 3
[0065] Embodiment 3: Compound Amino Acid Injection I:
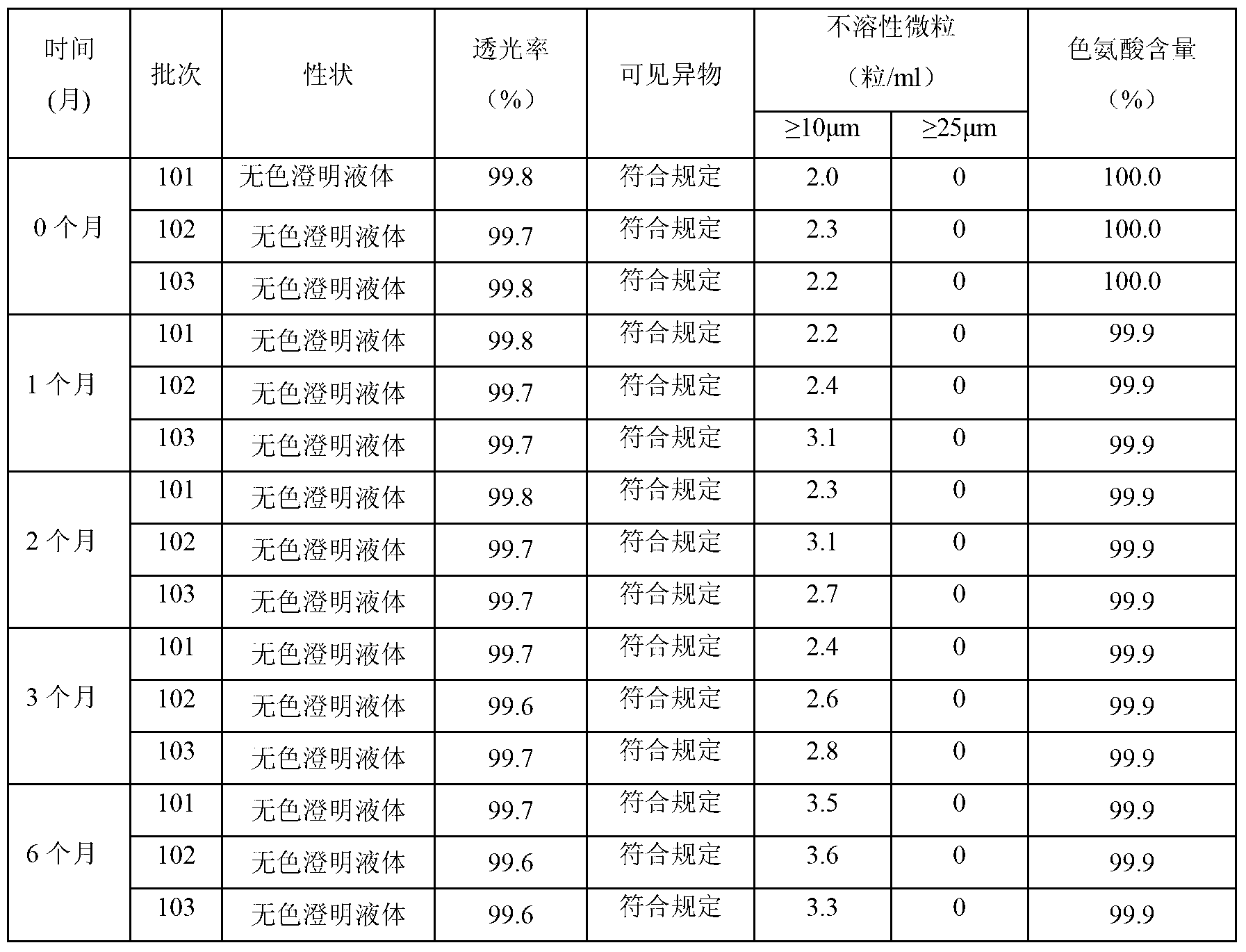
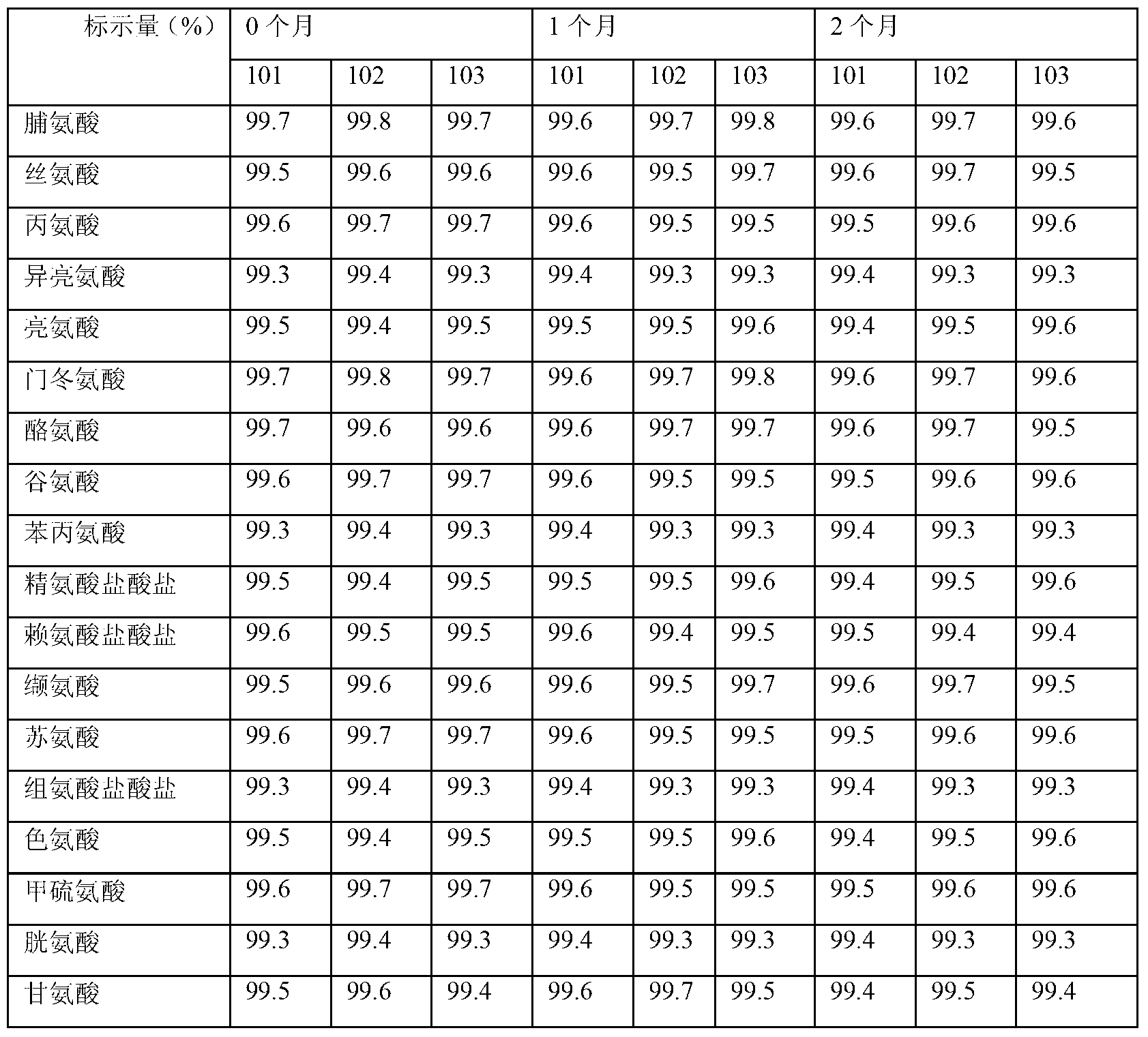
[0066] Each 1000ml contains: glutamic acid 9.0g, proline 8.1g, serine 7.5g, phenylalanine 5.5g, leucine 5.3g, valine 4.3g, aspartic acid 4.1g, isoleucine Acid 3.9g, Lysine hydrochloride 4.9g, Arginine 3.3g, Threonine 3.0g, Alanine 3.0g, Histidine 2.4g, Glycine 2.1g, Methionine 1.9g, Cysteine Salt 0.145g, tryptophan compound 1.0g, tyrosine 0.5g; calcium chloride (CaCl 2.2H 2 O) 0.368g, potassium chloride (KCl) 0.375g, magnesium sulfate (MgSO 4 .7H 2 O) 0.37g, sodium hydroxide (NaOH) 2.0g, potassium hydroxide (KOH) 0.84g; sorbitol 20g, sodium bisulfite 0.01g.
[0067] The preparation method is:
[0068] 1. prepare tryptophan compound according to embodiment 1 method;
[0069] 2. Liquid preparation: Weigh the remaining 17 kinds of amino acids in proportion, add them to water for injection, then add sorbitol and sodium bisulfite to the above solution in proportion, raise the temperature to 40°C, add water for injection ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com