Preparation method of high-optical purity bortezomib and intermediate of bortezomib
A bortezomib and optical purity technology, applied in the field of drug synthesis, can solve the problems of difficult removal of isomers and unsatisfactory chiral purity, and achieve the effect of high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
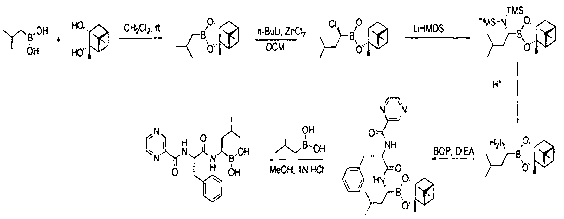
[0070] Example 1: Synthesis of compound of formula II
[0071] Add 6.2L of tetrahydrofuran to a 10L clean and dry reactor, add 232.9g of isobutylboric acid and 600g of formula I compound in sequence, react at room temperature for 16 hours, and distill under reduced pressure to obtain a colorless and transparent liquid, and add 8L of n-hexane to it , Washed once with 3L of saturated sodium chloride, collected the organic phase, dried over anhydrous sodium sulfate, and distilled under reduced pressure until there was no dripping liquid to obtain 599 g of colorless oil with a yield of 100% and a GC content of 99.8%.
[0072]
Embodiment 2
[0073] Example 2: Synthesis of compound of formula III
[0074] Under nitrogen protection, add 6L of tetrahydrofuran, 500g of formula II compound into a 10L clean and dry reactor, cool to -65°C, add 855ml of lithium diisopropylamide (2mol / L) dropwise for 2-3 hours, after the addition is complete , Continue the reaction for 30 minutes, add 350g of anhydrous zinc chloride in batches, after the addition, continue to react for 30 minutes, place at room temperature to continue the reaction for 8 hours, the reaction is over, the reaction is concentrated to no dripping liquid, add 4L saturated chlorinated The reaction was quenched by ammonium and extracted twice by adding 8L of n-hexane, washed with 4L of saturated sodium chloride solution, combined the organic phases, dried with 2kg of anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a pale yellow oil, 524g, yield, 90 %, GC content 92%.
[0075] 1 H NMR (CDCl 3 , 400MHz) δ 0.80-0.83 (m, 6H), 0.91-1.89 (m, 25H)...
Embodiment 3
[0076] Example 3: Synthesis of formula IV compound hydrochloride
[0077] Under nitrogen protection, add 4.8L of tetrahydrofuran, 500g of compound of formula III to a 10L clean and dry reaction kettle, cool to -65℃, dropwise add 1614ml (bistrimethylsilylamino) lithium (1mol / L), 2-3 hours After the dripping is completed, place at room temperature to continue the reaction for 6 hours. After the reaction is completed, the reaction is concentrated to no dripping liquid, a large amount of yellow solid is precipitated by adding n-hexane, filtered, the filtrate is concentrated to dryness, and 2L dioxane is added 4L 2N ether hydrogen chloride was added dropwise at -5~5℃, reacted for 12 hours, filtered, the filtrate was concentrated to no dripping liquid, a large amount of white solid was precipitated by adding n-hexane, filtered and dried to obtain the hydrochloric acid of formula IV compound Salt 417.9g, the yield is 80%.
[0078] 1 H NMR (DMSO- d 6 , 400MHz) δ 0.80-0.85 (m, 6H), 0.98-1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com