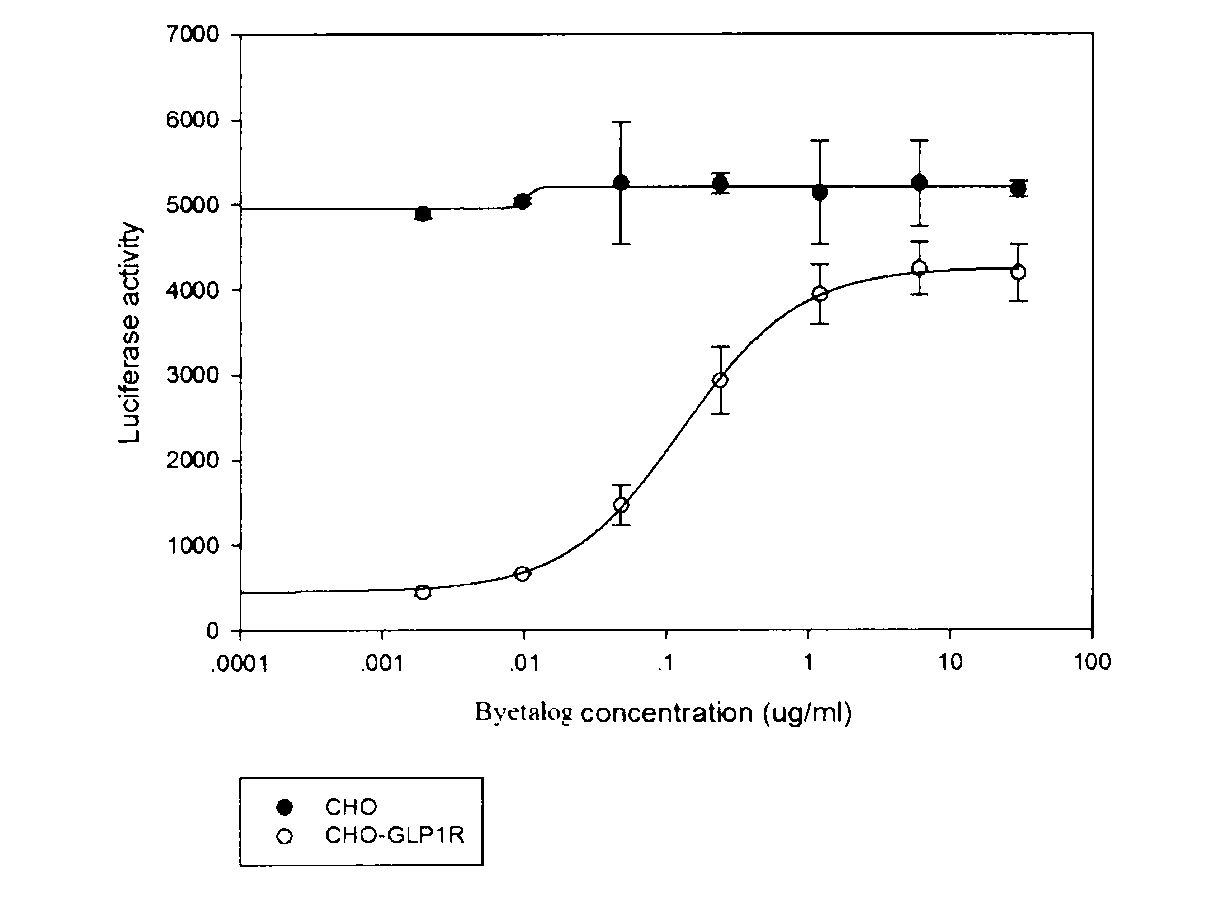
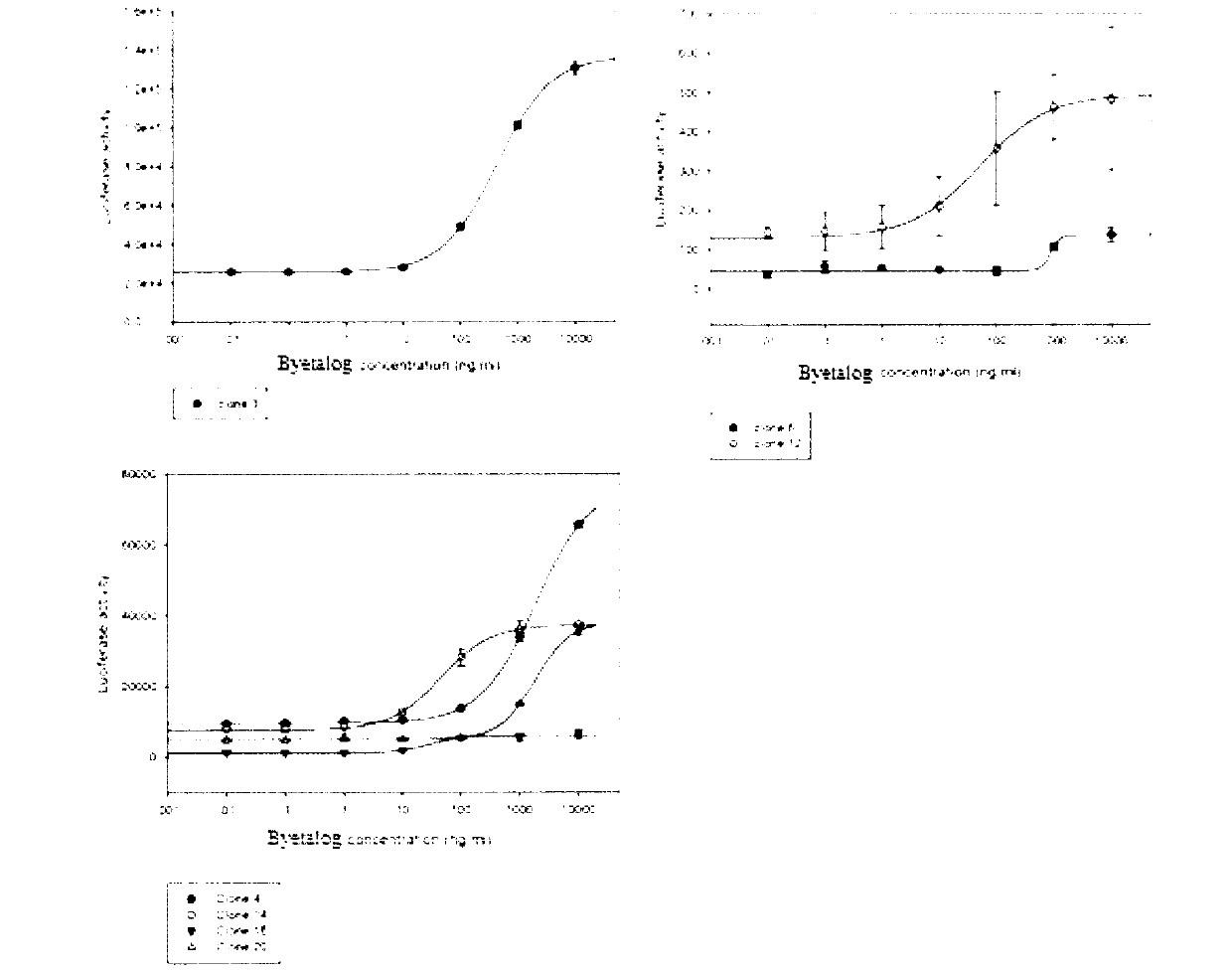
RGA (Report Gene Assay) method for detecting biological activity of exendin-4-HAS Byetalog
An insulin-stimulating and fusion protein technology, applied in biological testing, material testing products, cells modified by introducing foreign genetic material, etc., can solve the problems of cumbersome ELISA detection steps, expensive and other problems, and achieve objective and reliable results, small variation, high The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] 1. Materials and methods:
[0022] 1.1 Research Object: Insulin Secretory Stimulating Peptide Fusion Protein
[0023] 1.2 GLP-1R plasmid: purchased from Aurui Dongyuan Gene Co., Ltd.
[0024] pGL4.26 vector: purchased from promega.
[0025] CHO-K1 cells: purchased from ATCC.
[0026] Byetalog standard products and samples: Retained by the Recombinant Technology Product Office of China Institute of Food and Drug Research and Testing.
[0027] 1.3 Reagents:
[0028] Growth medium: F12K+10% fetal bovine serum (GIBCO, #10099)+1% double antibody (GIBCO, #15240)
[0029] Plate medium: Opti-MEM (GIBCO, #31985)
[0030] MegaTran 1.0 transfection reagent: origene, TT200003
[0031] G418: CalBiochem, #345810
[0032] Hygromycin: Cellgro, 30-240-CR
[0033] Selection medium: growth medium + 200ug / ml G418 + 300ug / ml hygromycin
[0034] Glo analysis: promega, E2661
[0035] Luciferase substrate: promega, E2650
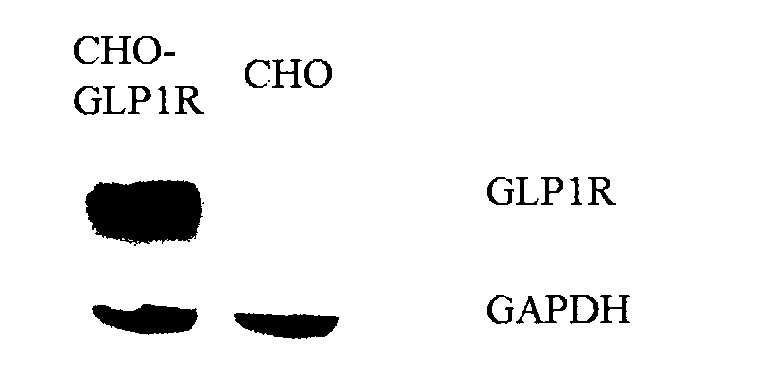
[0036] GLP-1R antibody: Abcam, ab39072
[0037] Chemilumine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com