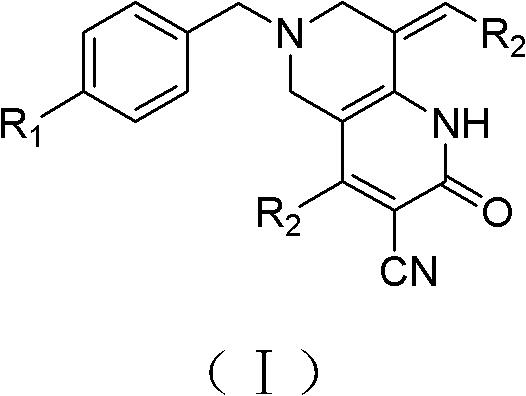
Tetrahydropyridopyridone derivative as well as preparation method and application thereof
A kind of technology of tetrahydropyridopyridone and tetrahydropyridine, which is applied in the field of tetrahydropyridopyridone derivatives and preparation thereof, and can solve the problems of lack of leading compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of 6-(4-fluorobenzyl)-4-(2-chlorophenyl)-8-(2-chlorobenzylidene)-3-cyano-1,2,5,6-tetra Hydropyrido[4,3-b]pyridin-2-one (Ia).
[0042]
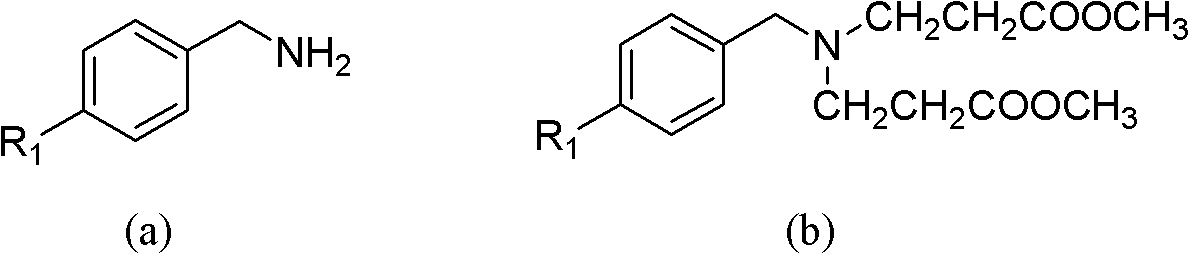
[0043] At room temperature, add 0.16mol methyl acrylate and 7mL methanol into a 100mL three-necked flask, and slowly add a mixture of 0.04mol p-fluorobenzylamine and 4mL methanol into the three-necked flask while stirring, so that the temperature of the reaction system does not exceed 50°C . After the dropwise addition, heat to reflux for 8 hours. After the reaction is over, recover methanol and unreacted methyl acrylate, and distill under reduced pressure to obtain light yellow oily liquid N,N-bis(methoxycarbonylethyl)-p-fluorobenzylamine (b 1 ).
[0044] Add 15mL of anhydrous toluene and 0.122mol of sodium metal to a 250mL dry three-necked flask, stir and heat to reflux, add 0.2mL of anhydrous methanol, and then slowly add 0.04mol of N,N-bis(methoxycarbonylethyl)-p-fluorine Benzylamine (b 1 ) and 20mL of...
Embodiment 2
[0048] Example 2: Preparation of 6-(4-fluorobenzyl)-4-(4-methylphenyl)-8-(4-methylbenzylidene)-3-cyano-1,2,5,6-tetrahydro Pyrido[4,3-b]pyridin-2-one (Ib).
[0049]
[0050] Prepare N-p-fluorobenzyl-4-piperidone (d) in the same manner as in Example 1 1 ).
[0051] Add 0.005mol N-p-fluorobenzyl-4-piperidone (d 1 ) and 0.01mol p-tolualdehyde, add 15mL absolute ethanol, stir and add 1mL 10% NaOH (mass fraction), stir at room temperature for 30min, a yellow solid is precipitated, and the reaction process is tracked by thin layer chromatography (TLC). After the reaction was finished, the solid was washed with ethanol, and recrystallized with ethyl acetate and petroleum ether to obtain N-(4-fluorobenzyl)-3,5-bis-p-methylbenzylidene piperidin-4-one (e 2 ).
[0052] Add N-(4-fluorobenzyl)-3,5-bis-p-methylbenzylidenepiperidin-4-one (e 2 ) (1mmol), malononitrile (1.5mmol, 99mg), ammonium acetate (1.5mmol) absolute ethanol (6mL) was heated under reflux for 8 hours, the reflux temp...
Embodiment 3
[0054] Example 3: Preparation of 6-(4-fluorobenzyl)-4-(4-fluorophenyl)-8-(4-fluorobenzylidene)-3-cyano-1,2,5,6-tetra Hydropyrido[4,3-b]pyridin-2-one (Ic).
[0055]
[0056] Prepare N-p-fluorobenzyl-4-piperidone (d) in the same manner as in Example 1 1 ).
[0057] Add 0.005mol N-p-fluorobenzyl-4-piperidone (d 1 ) and 0.01mol p-fluorobenzaldehyde, add 15mL absolute ethanol, stir and add 1mL 10% NaOH (mass fraction), stir at room temperature for 30min, a yellow solid is precipitated, and the reaction process is tracked by thin layer chromatography (TLC). After the reaction was finished, the solid was washed with ethanol, and recrystallized with ethyl acetate and petroleum ether to obtain N-(4-fluorobenzyl)-3,5-bis-p-fluorobenzylidene-4-piperidone (e 3 ).
[0058] Add N-(4-fluorobenzyl)-3,5-bis-p-fluorobenzylidenepiperidin-4-one (e 3 ) (1mmol), malononitrile (1.5mmol, 99mg), ammonium acetate (1.5mmol) absolute ethanol (6mL) was heated under reflux for 8 hours, the reflux t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com