Small-size sodium ozagrel freeze-dried powder needle as well as preparation method and production device thereof
A technology for sodium ozagrel and freeze-dried powder injection, which is applied in the field of small-volume sodium ozagrel freeze-dried powder injection and its preparation, can solve the problems of poor stability of ozagrel, shortened product validity period, poor product reconstitution, and the like, Achieve the effect of reducing packaging and transportation costs, reducing volume, and optimizing product processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
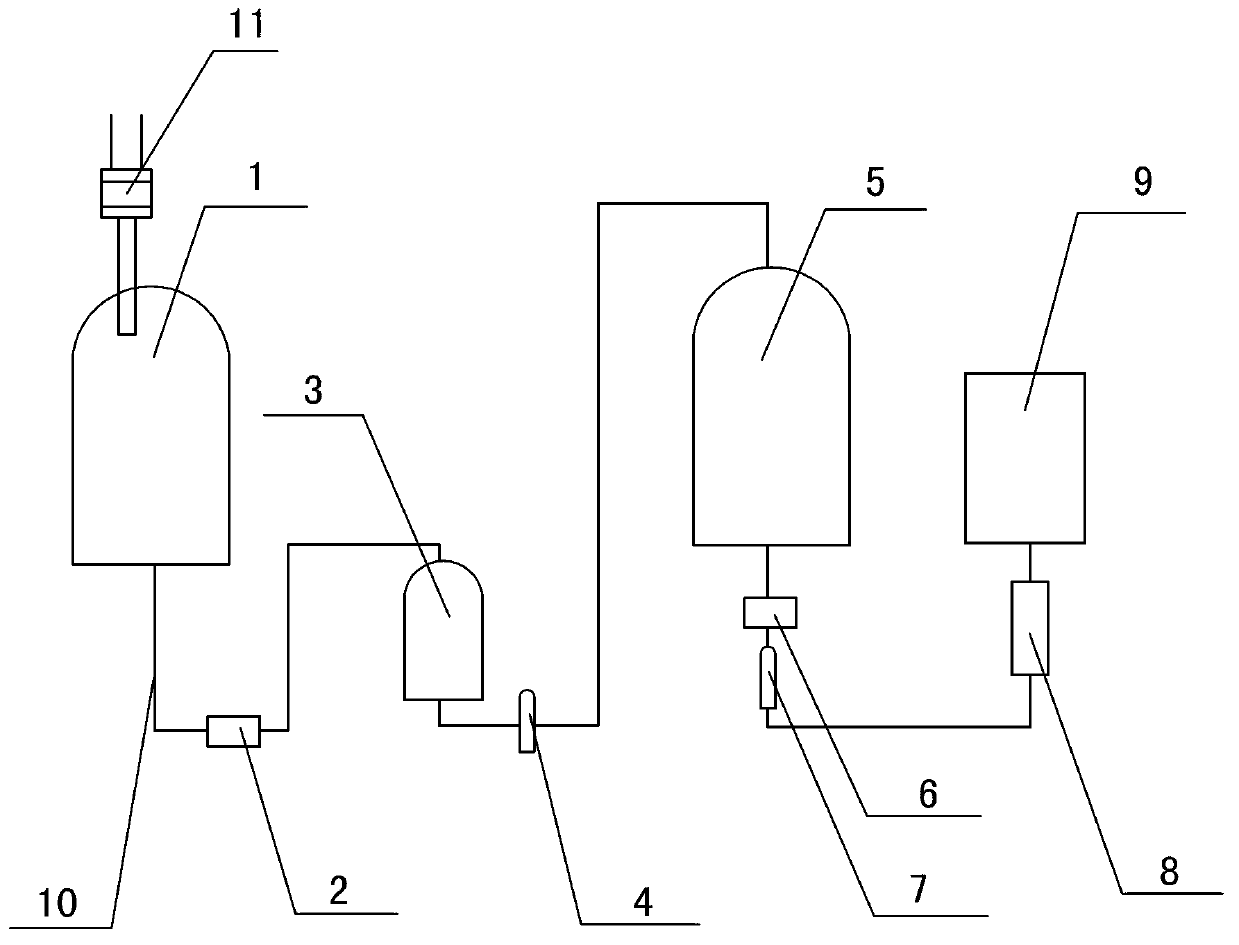
Image
Examples
Embodiment 1
[0030] The preparation method of 20mg small volume ozagrel sodium freeze-dried powder injection:
[0031] The formula is as follows:
[0032]
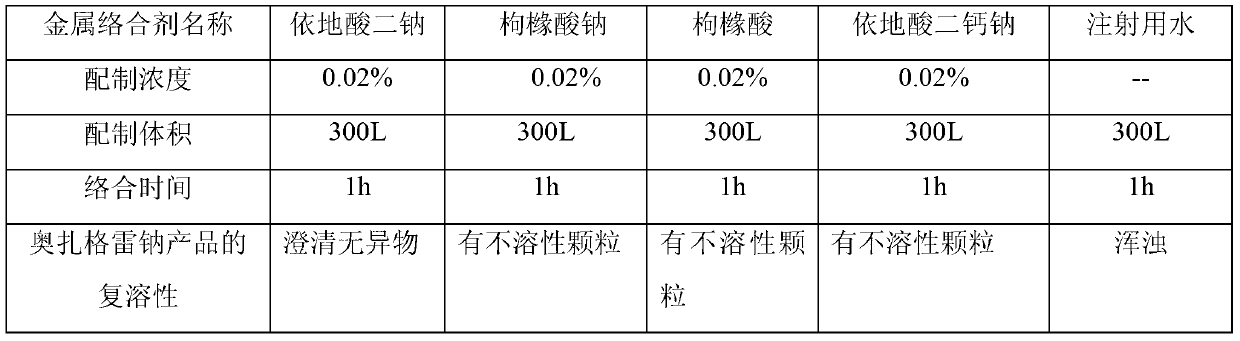
[0033] Before production, flush the device with edetate disodium with a concentration of 0.018%, complex for 0.8h, rinse with water for injection after the complexation is completed, and then prepare the nitrogen gas through the nitrogen sterilization filter to replace the feed liquid with air, which is called Take the prescribed amount of sodium citrate and edetate disodium, add them into the container and start stirring, take the prescribed amount of ozagrel and sodium hydroxide, the salt formation reaction is complete, adjust the pH to 8.0, and control the temperature at 18°C. After the preparation is completed, weigh 1.28g of activated carbon and add it, stir and absorb for 25 minutes, turn on the first Grundfos pump and use a titanium rod filter to decarbonize and filter, and the feed liquid is sterilized by the first sterilizi...
Embodiment 2
[0037] The preparation method of 40mg small volume ozagrel sodium freeze-dried powder injection:
[0038]
[0039] Before production, flush the device with edetate disodium with a concentration of 0.02%, complex for 1 hour, rinse with water for injection after the complexation is completed, and then prepare it. Nitrogen passes through the nitrogen sterilization filter to replace the feed liquid with air, and weighs Add the prescribed amount of sodium citrate and edetate disodium into the container and start stirring, take the prescribed amount of ozagrel and sodium hydroxide, the salt formation reaction is complete, adjust the pH to 9.3, and control the temperature at 20°C, the preparation is completed Finally, weigh 2g of activated carbon and add it, stir and absorb for 30min, turn on the first Grundfos pump and use a titanium rod filter to decarbonize and filter, and pass the feed liquid through the first sterilizing filter element to sterilize and transport it to the asep...
Embodiment 3
[0042] The preparation method of 80mg small volume ozagrel sodium freeze-dried powder injection:
[0043]
[0044] Before production, flush the device with edetate disodium with a concentration of 0.022%, complex for 1.2 hours, rinse with water for injection after the complexation is completed, and then prepare the nitrogen gas through the nitrogen sterilization filter to replace the feed liquid with air, which is called Take the prescribed amount of sodium citrate and edetate disodium, add them into the container and start stirring, take the prescribed amount of ozagrel and sodium hydroxide, the salt formation reaction is complete, adjust the pH to 9.5, and control the temperature at 19°C to prepare After completion, weigh 2.46g of activated carbon and add it, stir and absorb for 28 minutes, turn on the first Grundfos pump and use a titanium rod filter to decarbonize and filter, and the feed liquid is sterilized by the first sterilizing filter element and transported to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com