Diimmonium-based component and near infrared absorption filter using same
A compound, diimine technology, applied in the field of diimine-based compounds and near-infrared absorption filters using the compound, can solve the problem of product weather resistance, and is not suitable for use in places closely related to human living environments , Reduced product durability and other issues, to achieve excellent durability and weather resistance, excellent light transmittance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
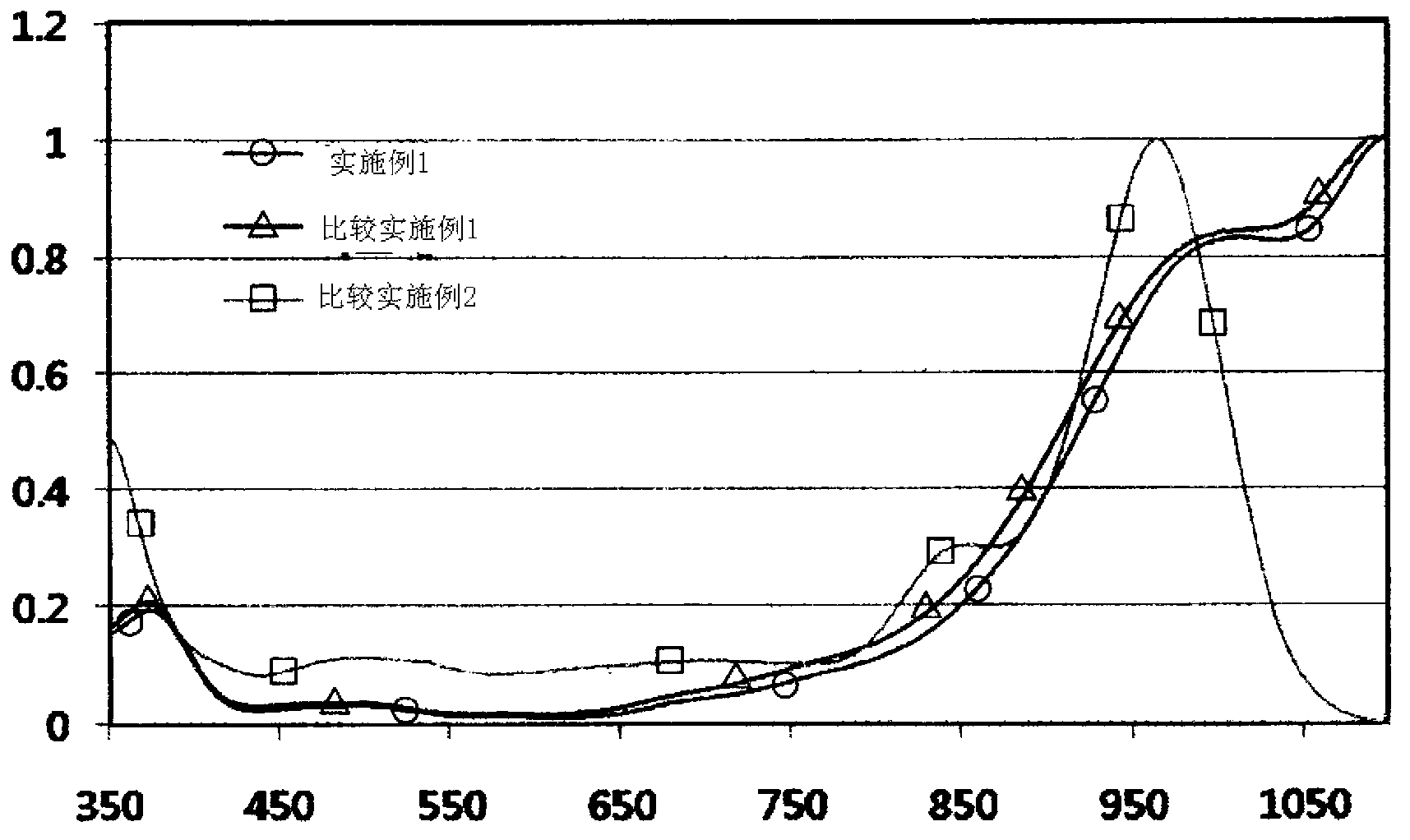
Examples
Embodiment 1
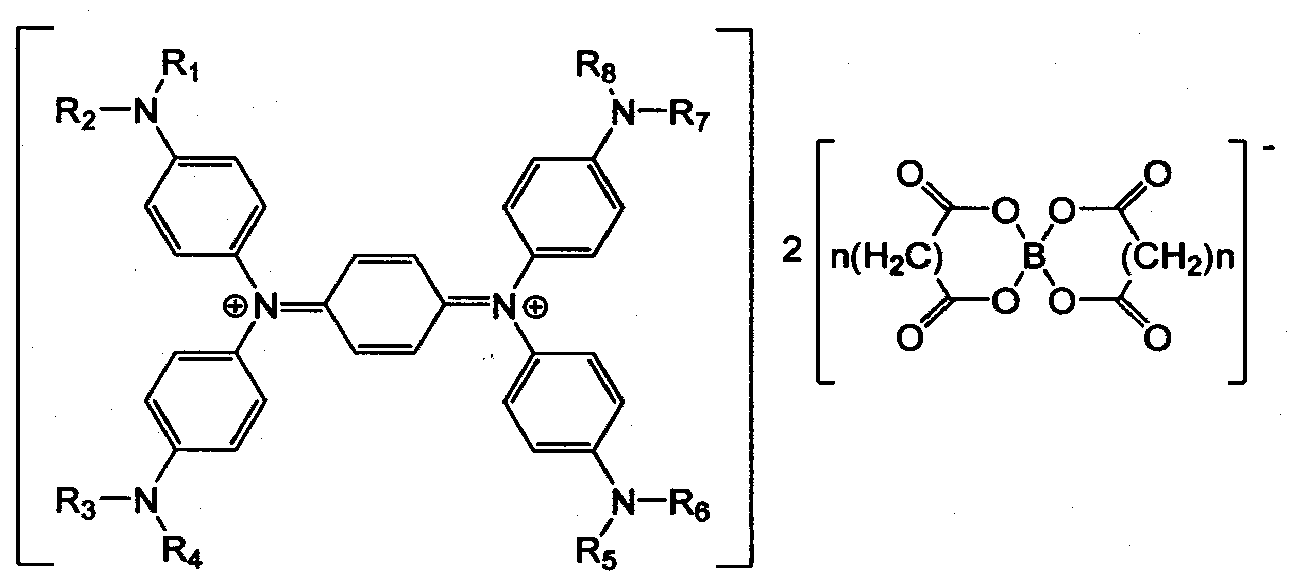
[0028] Example 1: Preparation of diimine-based compound (N, N, N', N'-tetrakis(p-diisobutylaminobenzene) p-phenylenedimine (N, N, N', N'-tetrakis( p-diisobutylaminophenyl)-p-phenylene diimmonium)
[0029] 12g N,N,N',N'-tetrakis(4-aminophenyl)-1,4-p-phenylenediamine (N,N,N',N'-tetrakis(4-aminophenyl)-1,4- Phenylenediamine), 40g isobutylbromide and 18g sodium bicarbonate were added to a three-necked flask with a reflux device, and then 30g N-methylpyrrolidone (N-methylpyrrolidone) was added, and the mixture was stirred and reacted at 80°C for 9 hours . After the reaction was completed, 300 g of dichloromethane and 1 L of water were added to the flask, followed by stirring for 30 minutes. After stirring, distill the separated dichloromethane layer with a vacuum distiller to obtain 30 g of N,N,N',N'-tetrakis(p-diisobutylaminobenzene)-p-phenylenediamine (primary reactant).
[0030] 30 g of the primary reactants were added to a 3-necked flask with a reflux device, and then 15 g o...
Embodiment 2
[0031]Example 2: Preparation of a diimine-based compound (N,N,N',N'-tetrakis(p-diisobutylaminobenzene)-p-phenylenedimine bismalonate boronic acid)
[0032] 30g of N,N,N',N'-tetrakis(p-diisobutylaminobenzene-p-phenylenediamine) obtained by the method of Example 1, 15g of lithium bismalonatoborate, 150g of dichloro Methane and 60g ethanol are added in the flask, and the mixture is refluxed for 2 hours. Then add 10g sodium persulfate and 200g water, and reflux for 2 hours. After the reflux is completed, add 250g methylene chloride and 300g water in the reaction flask, then use vacuum distillation The separated methylene chloride layer was distilled to obtain 8g bismalonic acid borate N,N,N',N'-tetrakis(p-diisobutylaminobenzene)p-phenylenedimine (biso(xalato)borate N,N ,N',N'-tetrakis(p-diisobutylaminophenyl)-p-phenylenediimmonium), that is, a diimine-based composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| UV absorption wavelength | aaaaa | aaaaa |
| UV absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com