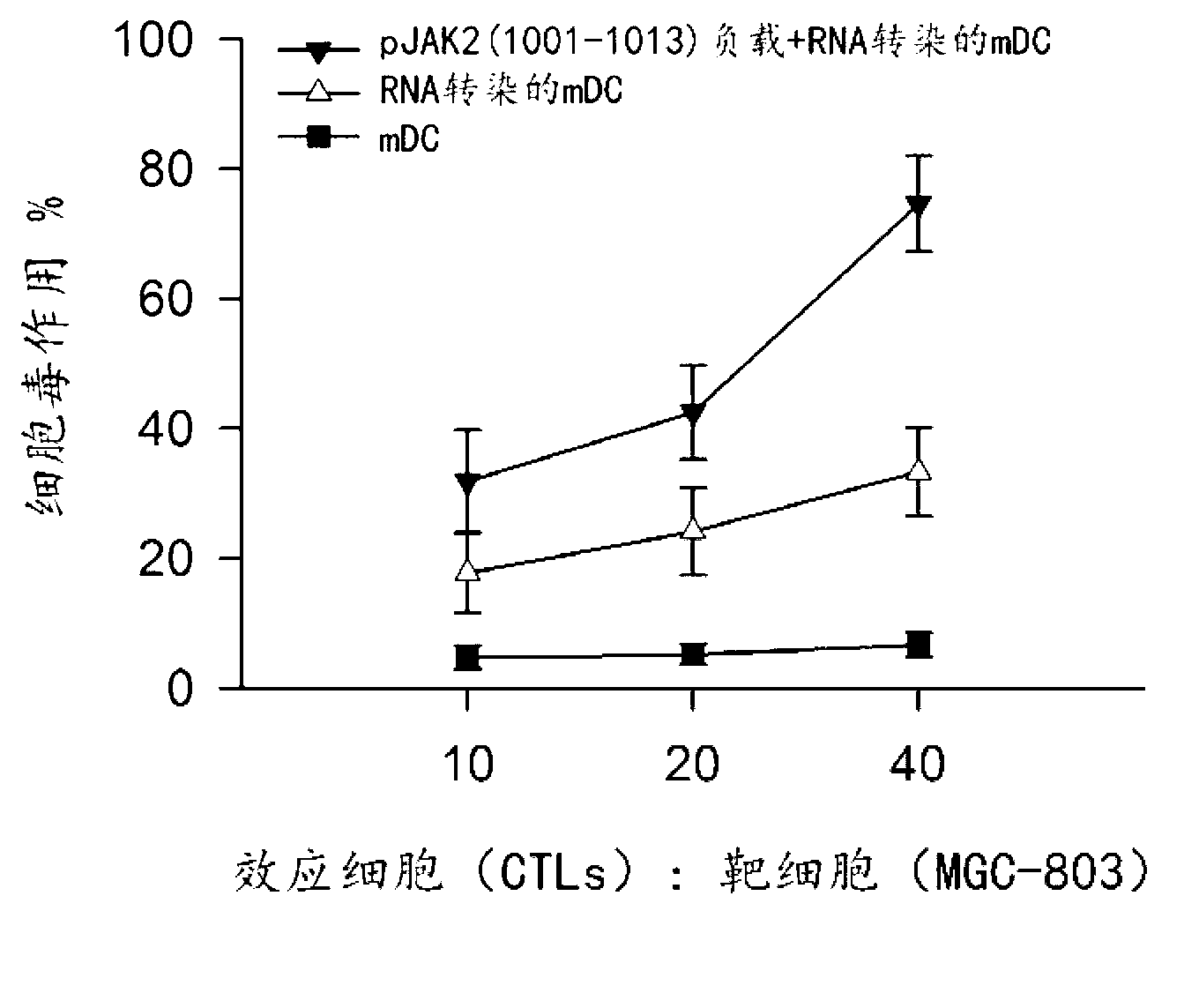Method for preparing dendritic cells for effectively submitting gastric cancer antigens
A technology for dendritic cells and gastric cancer cells, applied in the field of preparation of dendritic cells, can solve the problems that the preparation methods have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Extraction and identification of gastric cancer cell MGC-803 total RNA
[0029] 1.1 Extraction of total RNA from gastric cancer
[0030] (1) Place the culture dish covered with about 90% of the gastric cancer cell MGC-803 on the bottom wall on ice, and absorb the culture solution;
[0031] (2) Add 1ml directly Reagent, pipetting repeatedly;
[0032] (3) Transfer the cell lysate to a new 1.5ml Eppendorf tube and let it stand at room temperature for 5 minutes;
[0033] (4) Add 0.2ml chloroform and mix vigorously for 15s;
[0034] (5) Place at room temperature for 2 to 3 minutes;
[0035] (6) Centrifuge at 12,000rpm, 4°C, 15min;
[0036] (7) See the upper water phase, the middle mixed phase, and the lower phenolic phase. Aspirate the aqueous phase and transfer to a new Eppendorf tube, about 0.5ml;
[0037] (8) Add 0.5ml of isopropanol, mix by inverting, and place at room temperature for 10 minutes;
[0038] (9) Centrifuge at 12,000rpm, 4°C, 10min;
[003...
Embodiment 2
[0051] Example 2: Synthesis of SOCS1 antagonist pJAK2(1001-1013) polypeptide
[0052] The amino acid sequence of the pJAK2(1001–1013) polypeptide is 1001 LPQDKE Y YKVKE 1013 , where the underscore Y Phosphorylated tyrosine. The peptide was synthesized by Shanghai Jill Biochemical Company with a purity of over 98.0%. In order to prevent the degradation of the peptide during in vitro manipulation and ensure efficient entry into DC, a lipophilic group (palmitoyl-lysine) was added to the N-terminus of the peptide during synthesis.
Embodiment 3
[0053] Example 3: Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
[0054] Freshly collected 50ml of anticoagulated peripheral blood (20U heparin sodium / ml whole blood) from healthy volunteers, diluted it with 37°C PBS, and slowly poured it into the centrifuge containing the rewarmed Ficoll-Hypaque lymphocyte separation medium along the tube wall. tube (blood:separation fluid=1:1) to make the interface clear. Gradient centrifugation (room temperature, 800g, 20min), take the middle buffy coat cell layer, put it into a 50ml centrifuge tube, suspend the cells with PBS without calcium and magnesium, centrifuge (800g, 10min), wash the cells twice, and centrifuge again (1200rpm , 10 min), and the PBMCs were obtained after the platelets were washed out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com