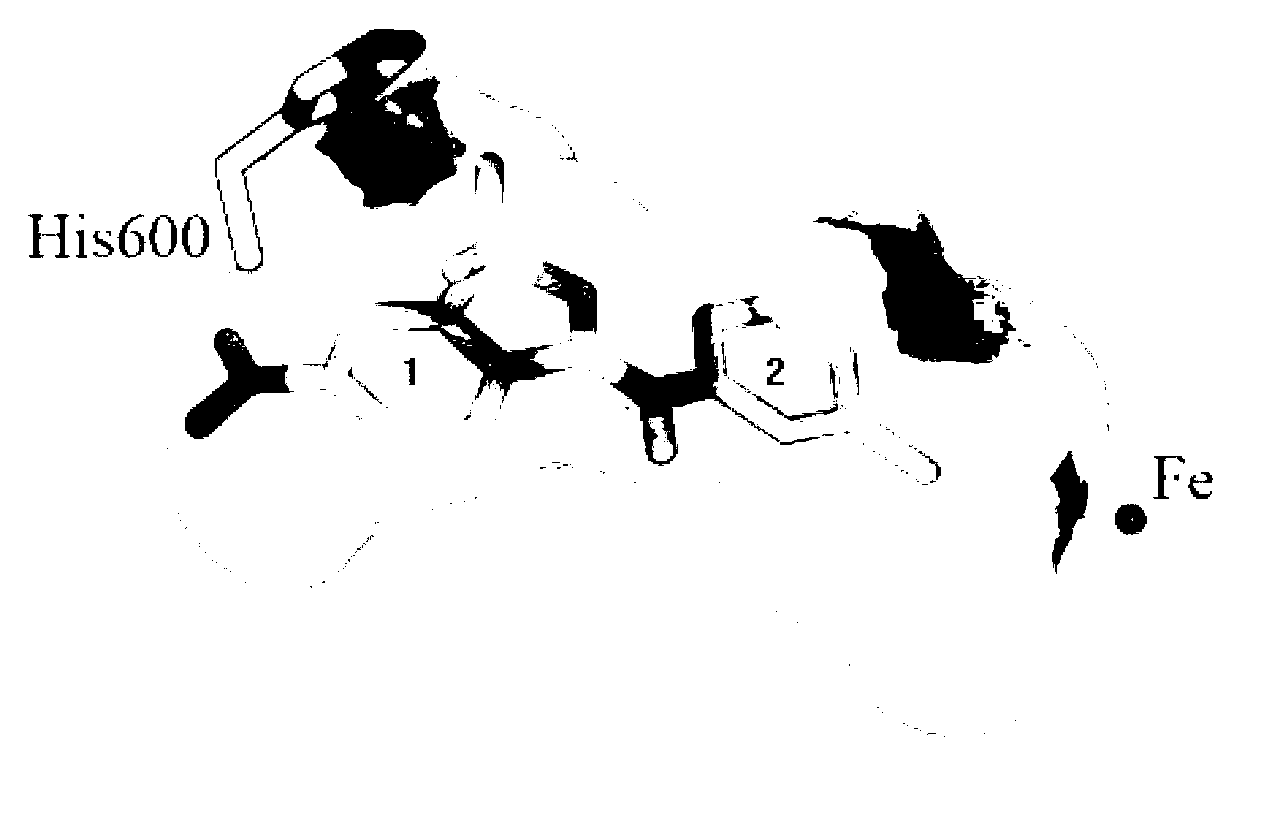
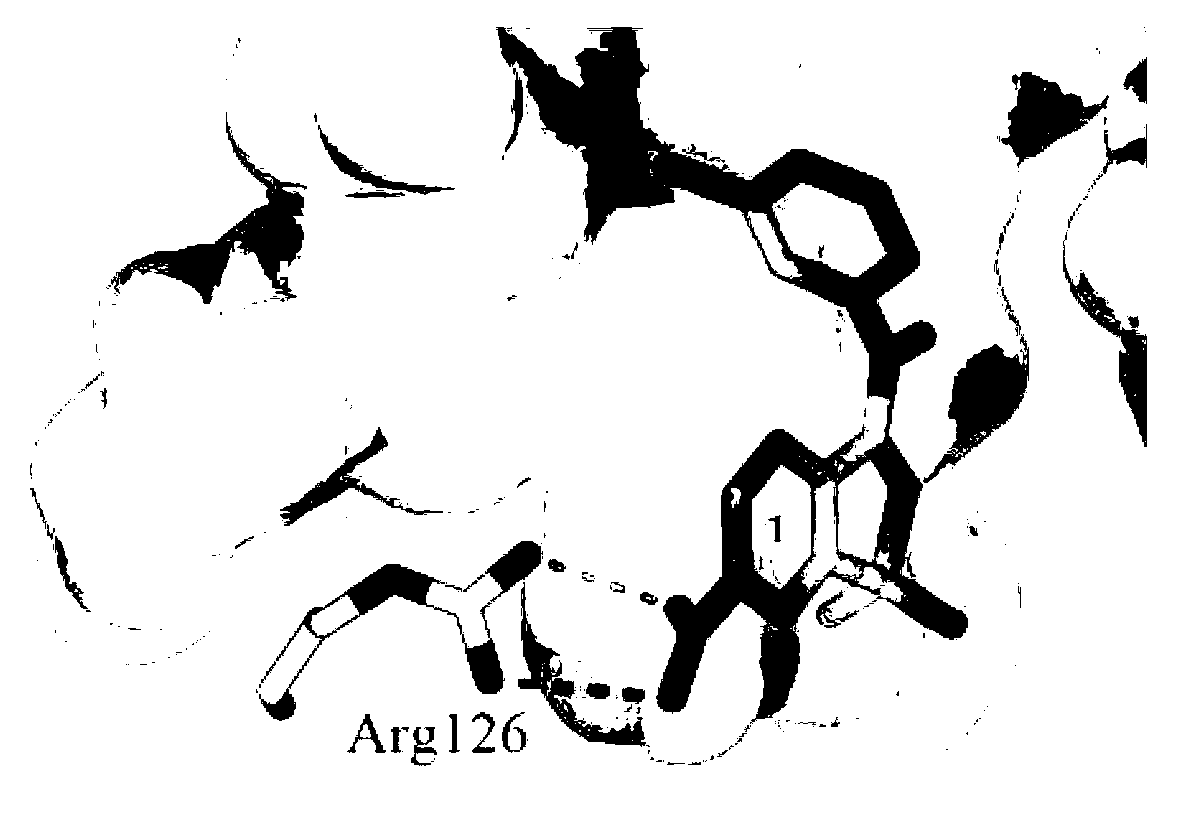
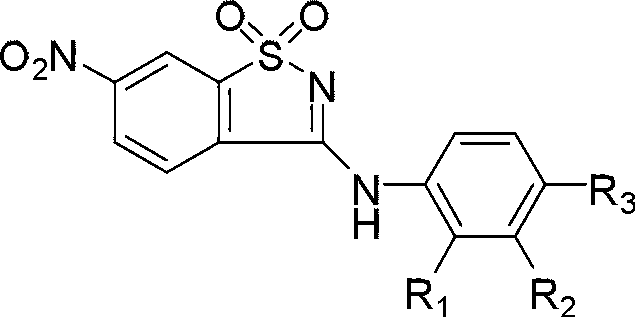
Benzisoxa thiazoles 5-LOX and mPGES-1 inhibitor and application
A technology of benzisothiazoles and isothiazoles, used in the preparation of medicines for the treatment and prevention of various inflammations, the field of benzisothiazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0045] Embodiment 2, the synthesis of bifunctional inhibitor
[0046] The synthesis method of this bifunctional inhibitor is described below by taking the compound 3-(3-methylphenyl)amino-6-nitro-1,1-dioxybenzisothiazole (PKUMDL_AAL_101) as an example.
[0047]
[0048] Reagents and conditions: (a) SOCl 2 , DMF(cat.), Dioxane, 110℃, 1.5h; (b) NEt3, THF, rt, 4~6h.
[0049] Under the protection of argon, add 0.23g (1mmol) 6-nitro-1,1,3-trioxo-2,3-dihydro-benzisothiazole and 10mL 1,4- Dioxane (Dioxane), after stirring to dissolve the solid completely, add 0.36g (3mmol) thionyl chloride (SOCl 2 ) in 0.5mL dimethylformamide (DMF) solution, heated to reflux at 110°C for 1.5h, the reaction was completed, and the solvent and excess thionyl chloride were distilled off under reduced pressure to obtain the intermediate 3-chloro-6-nitro-1, 1-Dioxo-benzisothiazole was directly carried on to the next reaction without purification. 15 mL of tetrahydrofuran, 0.3 g (3 mmol) of triethyla...
Embodiment 3
[0062] Embodiment 3, the in vitro activity of 5-LOX is measured by fluorescence spectrophotometry
[0063] The principle of fluorescence spectrophotometry is based on the reaction intermediate product 5-HPETE of 5-LOX, which can convert the fluorescent chromogenic agent H 2 DCFDA is oxidized to generate a highly fluorescent molecular DCF with an excitation wavelength of 500nm and an emission wavelength of 520nm. When measuring the activity, first add the 5-LOX enzyme to the assay buffer (50mM Tris-HCl, pH 7.5, 0.2mM ATP, 0.1mM dithiothreitol (dithiothreitol, DTT), 0.1mM EDTA, 0.5mM CaCl 2 ), incubate in a 96-well plate at 25°C for 10 min to equilibrate. Add color developer H 2 DCFDA (final concentration of 10 μM) and arachidonic acid AA substrate (final concentration of 25 μM) initiated the reaction, and the generation of fluorescent product DCF was monitored over time with a fluorescent microplate reader (excitation wavelength was 500 nm, emission wavelength was 520nm). T...
Embodiment 4
[0065] Embodiment 4, the in vitro activity of mPGES-1 measured by enzyme-linked immunosorbent assay
[0066] The enzymatic activity of mPGES-1 was characterized by quantitative measurement of PGE2 produced by mPGES-1-catalyzed conversion of the substrate PGH2. The amount of catalyzed PGE2 was determined using a PGE2 ELISA kit (Cayman). For the determination method, see the kit instructions. When measuring the activity, the substrate PGH2 was first added to a 96-well plate at a constant temperature of 4°C. Add 100 μl of enzyme to initiate the reaction. After reacting at 4°C for 1 min, 150 μl of stop solution (50 mM FeCl2 and 100 mM citric acid) was added to terminate the reaction. After the solution was diluted, the content of the product PGE2 was determined using a PGE2 enzyme-linked immunosorbent assay kit. It should be noted that the substrate PGH2 is unstable and easy to decompose at high temperature, and should be placed in a constant temperature environment at 4°C dur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com