Method for preparing ferrite nano fiber
A nanofiber and ferrite technology, applied in fiber processing, spinning solution preparation, textile and papermaking, etc., can solve problems such as limiting fiber application, structural defects, affecting fiber crystal structure fiber surface structure, etc., to reduce dosage, The effect of improving magnetic performance and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
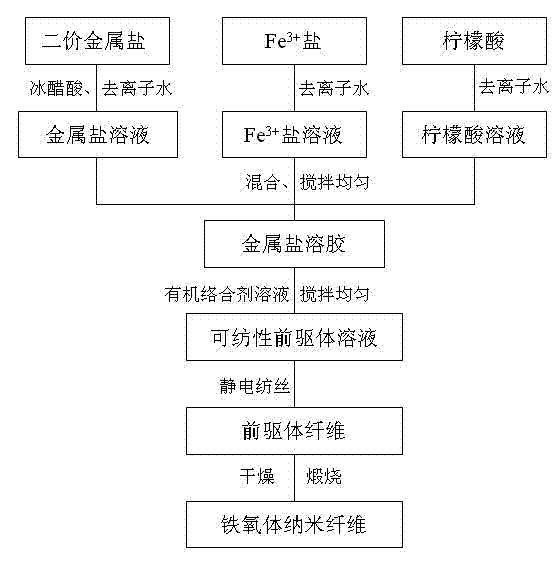
[0023] The process for preparing ferrite nanofibers is as follows: figure 1 shown.
[0024] Step 1: Add 0.50g of polyvinylpyrrolidone (PVP) into 4.5g of absolute ethanol, and stir the polymer for about 5 hours to completely dissolve the polymer to obtain a PVP ethanol solution. 27.2g ferric nitrate (Fe(NO 3 ) 3 9H 2 (2), 18.1g citric acid are dissolved in deionized water, 1.1g barium carbonate (BaCO 3 ) was dissolved in glacial acetic acid aqueous solution, and magnetically stirred for 1 hour until completely dissolved. The resulting metal salt solution was evaporated to a viscous sol in a rotary evaporator. Dissolve the obtained sol in the PVP ethanol solution, then add an appropriate amount of water to make the concentration of PVP 4wt%, and stir evenly to form a stable precursor solution;
[0025] Step 2: Transfer the obtained precursor solution to a plastic syringe with a stainless steel needle with an outer diameter of 0.6 mm. The distance between the needle and th...
Embodiment 2
[0028] Step 1: Add 1.0 g of polyvinylpyrrolidone (PVP) into 6.0 g of deionized water, and stir the polymer for about 5 hours to completely dissolve the polymer to obtain a PVP aqueous solution. 27.2g ferric nitrate (Fe(NO 3 ) 3 9H 2 (O), 19.5g citric acid, 2.87g barium nitrate (Ba(NO 3 ) 2 ) was dissolved in deionized water and magnetically stirred until completely dissolved. The resulting metal salt solution was evaporated to a viscous sol in a rotary evaporator. Dissolve the obtained sol in the PVP aqueous solution, adjust the concentration of PVP to 6wt%, and stir evenly to form a stable precursor solution;
[0029] Step 2: same as embodiment 1;
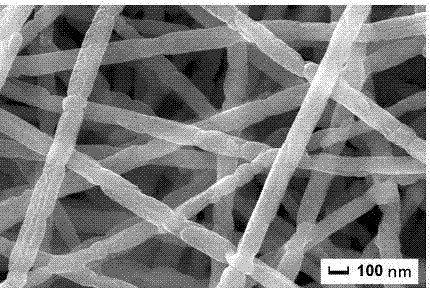
[0030] Step 3: Place the dried precursor fiber in an alumina crucible, raise the temperature to 800°C at a rate of 3°C / min in an air atmosphere, keep it warm for 2 hours, and naturally cool to room temperature to obtain a fiber with a diameter of about 100nm. The barium ferrite nanofibers, the SEM and magnetic properties of...
Embodiment 3
[0032] Step 1: Add 1.0 g of polyvinyl alcohol (PVA) into 6 g of absolute ethanol, and stir the polymer for about 5 hours to completely dissolve the polymer to obtain a PVA ethanol solution. 27.2g ferric nitrate (Fe(NO 3 ) 3 9H 2 (O), 14.5g citric acid, 2.87g barium nitrate (Ba(NO 3 ) 2 ) was dissolved in deionized water and magnetically stirred for 1 hour until completely dissolved. The resulting metal salt solution was evaporated to a viscous sol in a rotary evaporator. Dissolving the obtained sol in PVA ethanol solution, adding an appropriate amount of water so that the concentration of PVA is 8wt%, stirring evenly to form a stable precursor solution;
[0033] Step 2: same as embodiment 1;
[0034] Step 3: Place the dried precursor fiber in an alumina crucible, raise the temperature to 900°C at a rate of 3°C / min in an air atmosphere, keep it warm for 2 hours, and cool naturally to room temperature to obtain a fiber with a diameter of about 100nm. barium ferrite nanofi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com