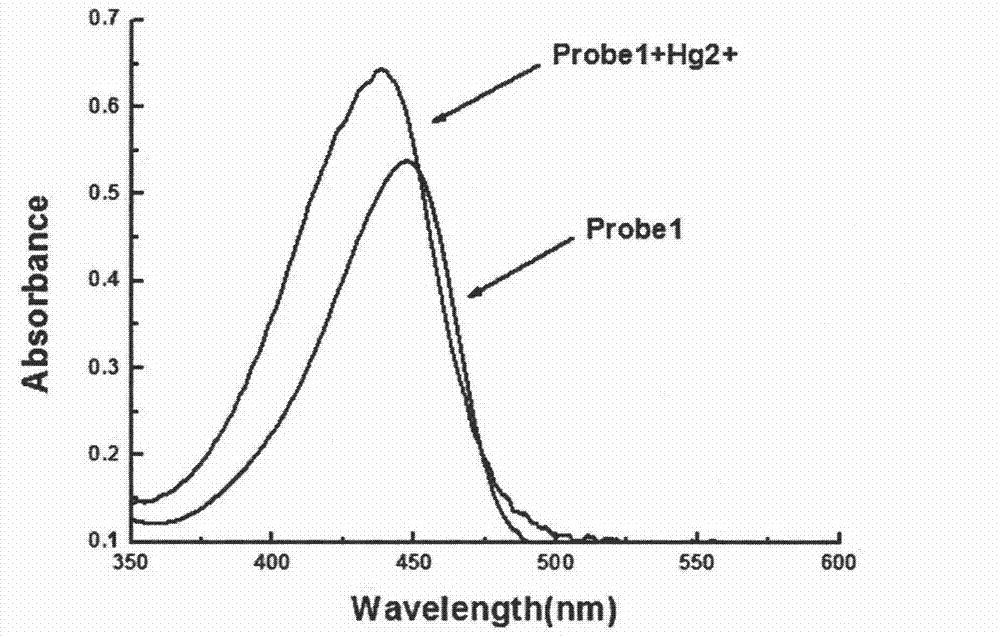
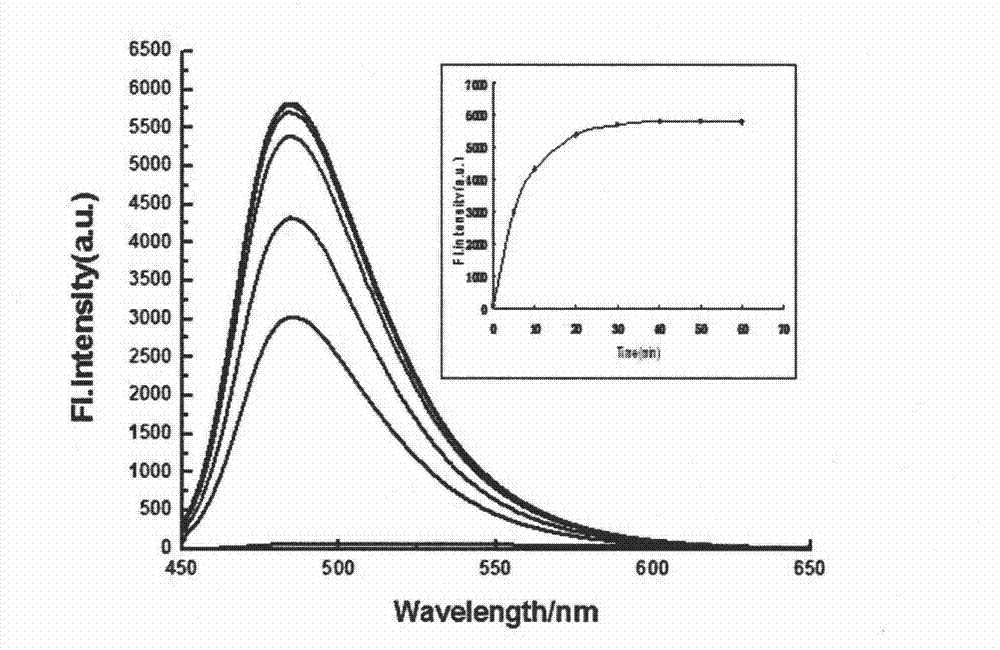
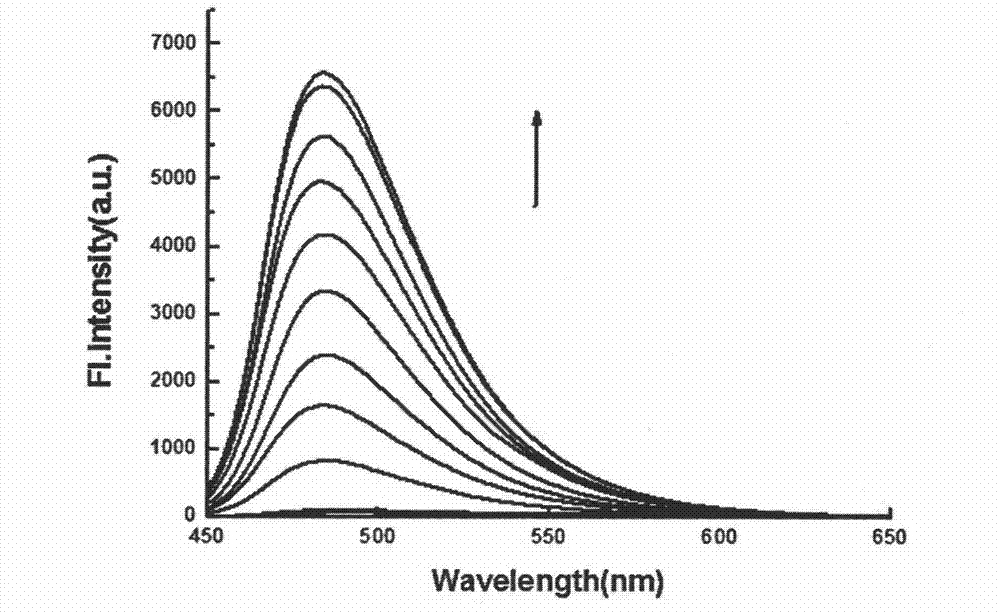
Synthesis and application of fluorescence enhancement detection mercury ion probe
A fluorescent molecular probe and fluorescent probe technology, applied in the field of chemical analysis and detection, can solve the problems of poor selectivity, easy to be interfered by other ions, etc., and achieve the effects of high synthesis yield, high sensitivity and fast response speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of 2-(2-bromoethoxy)-4-diethylaminobenzaldehyde
[0034] 4-Diethylamino salicylaldehyde (0.965g, 5mmol), potassium carbonate (1.383g, 20mmol) and 1,2-dibromoethane (9.4g, 50mmol) were added to 15mL of acetonitrile, and heated to reflux for 48h , cooled to room temperature, filtered to remove K 2 CO 3 , washed with acetonitrile, distilled off the solvent, and separated by column chromatography (petroleum ether at a volume ratio of 6:1: ethyl acetate was the eluent) to obtain 0.780 g (yield: 52%) of a pale yellow solid, namely 2- (2-Bromoethoxy)-4-diethylaminobenzaldehyde.
Embodiment 2
[0035] Embodiment 2: Preparation of 2-vinyloxy-4-diethylaminobenzaldehyde
[0036] Dissolve 2-(2-bromoethoxy)-4-diethylaminobenzaldehyde (300 mg, 1 mmol) in 10 mL of dry dimethyl sulfoxide, add potassium tert-butoxide (128 mg, 1.1 mmol) at 25 ° C The mixture was stirred for 4 hours and the progress of the reaction was monitored by thin layer chromatography. After the reaction, the reaction solution was poured into 70 mL of water, extracted twice with ethyl acetate (2×75 mL), the organic layer was separated, and washed with saturated ammonium chloride solution and saturated brine, respectively. The organic phase was separated and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and separated by column chromatography (petroleum ether:ethyl acetate at a volume ratio of 6:1 as eluent) to obtain 0.103 g (yield: 47%) of a light yellow oily liquid, which was 2-ethyleneoxy -4-Diethylaminobenzaldehyde.
Embodiment 3
[0037] Embodiment 3: Preparation of molecular fluorescent probe
[0038] Dissolve 2-vinyloxy-4-diethylaminobenzaldehyde (123 mg, 0.561 mmol) and malononitrile (75 mg, 1.137 mmol) in 7 mL of absolute ethanol, add piperidine (20 μL, 0.202 mmol), and After stirring for 5 hours, a light yellow solid was precipitated, which was filtered by suction and dried in air to obtain 113.8 mg of the product (yield: 72.09%), which was the probe molecule.
[0039] HNMR (400MHz, CDCl 3 ):δ ppm = 1.33 (6H, t, J = 8Hz), 3.47 (4H, q, J = 8Hz), 4.65 (1H, dd, J = 4, 1.6Hz), 4.92 (1H, dd, J = 1.6, 1.6Hz) , 6.22 (1H, d, J = 2.4Hz), 6.66 (1H, dd, J = 2.4, 2.4Hz), 6.95 (1H, dd, J = 6, 5.6Hz), 7.09 (1H, s), 8.06 ( 1H, d, J=9.6Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com