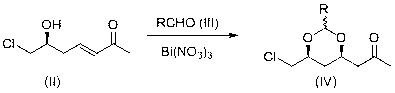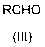Preparation method of 2-((4R, 6S)-6-chloromethyl-2-alkyl-1,3-dioxane -4-yl)acetic acid
A technology of dioxane and chloromethyl is applied in the field of synthesis of statin hypolipidemic drugs, can solve problems such as unfavorable large-scale industrial production, and achieve the effects of low cost, easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Will ( S )-7-Chloro-6-hydroxyhept-3-vin-2-one (1.82 g) was dissolved in dichloromethane (50 ml), and fresh acetaldehyde (2 ml) and nitric acid pentahydrate were added under stirring at room temperature Bismuth (0.5 g), stirred at room temperature in the dark for 4 d, after the reaction was completed, washed with water, dried the organic phase with anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid 1-((4 R ,6 S )-6chloromethyl-2-methyl-1,3-dioxan-4-yl)propan-2-one (2.00 g, 97%) = 4.8 o ( c 1.0, CHCl 3 );
[0045] 1 H NMR (400MHz, CDCl 3 ): δ 4.69 (q, J=4.8Hz, 1H) 4.05 (m, 1H) 3.81 (m, 1H) 3.49 (dd, J =11.2, 5.6Hz, 1H) 3.40 (dd, J =11.6, 5.2Hz, 1H) 2.73 (dd, J =16.4, 6.8Hz, 1H) 2.45 (dd, J =16.4, 5.2Hz, 1H) 2.13 (s, 3H) 1.68 (d, J =12.8Hz, 1H) 1.34~1.23 (m, 4H).
Embodiment 2
[0047] Will ( S )-7-Chloro-6-hydroxyhept-3-vin-2-one (1.82 g) was dissolved in 1,2-dichloroethane (50 ml), and propionaldehyde (1.5 g) and Bismuth nitrate pentahydrate (0.5 g), stirred at room temperature in the dark for 3 days, after the reaction was completed, washed with water, the organic phase was dried with anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid 1-((4 R ,6 S )-6chloromethyl-2-ethyl-1,3-dioxan-4-yl)propan-2-one (2.07 g, 94%) = 4.3 o ( c 1.0, CHCl 3 ).
Embodiment 3
[0049] Will ( S )-7-Chloro-6-hydroxyhept-3-vin-2-one (1.82 g) was dissolved in dichloromethane (50 ml), and freshly prepared acetaldehyde (4 ml) and pentahydrate were added under stirring at 0°C Bismuth nitrate (1 g), stirred in the dark at 0°C for 7 days, after the reaction was completed, washed with water, dried the organic phase with anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid 1-((4 R ,6 S )-6chloromethyl-2-ethyl-1,3-dioxan-4-yl)propan-2-one (1.96 g, 95%) = 4.7 o ( c 1.0, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com