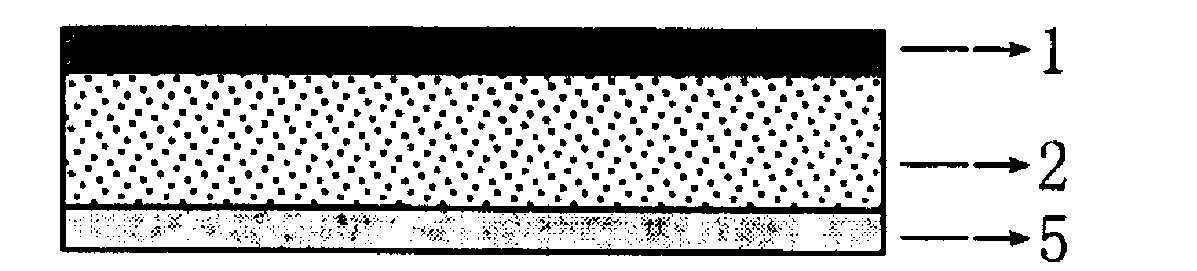
Granisetron and dexamethasone compound percutaneous controlled release patch and preparation method thereof
A technology of dexamethasone and compound recipe, applied in the field of pharmaceutical preparations, can solve the problems of unstable penetration, large difference in oil-water partition coefficient, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Prepare granisetron-dexamethasone compound transdermal controlled-release patch, which is composed of a backing layer, a pressure-sensitive adhesive skeleton-type drug-containing reservoir layer and a protective film. The preparation method is as follows:
[0039] (1) Accurately weigh 0.18g of granisetron, 0.48g of dexamethasone, 0.15g of succinic acid, add 6ml of ethanol and stir until dissolved, slowly add 1g of dibutyl sebacate and 0.16g of azone and stir until the solution becomes clear. Add 1.8g Eudragit E100, and continue to stir until the transparent gel-like drug-containing pressure-sensitive adhesive reservoir viscose;
[0040] (2) Heat the above-mentioned drug-containing pressure-sensitive adhesive reservoir viscose until the ethanol volatilizes to obtain a drug-containing glue with a suitable consistency, degas it by ultrasonic, and apply it on a certain area of CoTran by salivating process TM Put it on the 9720 polyethylene backing film, put it in an oven ...
Embodiment 2
[0055] Prepare granisetron-dexamethasone compound transdermal controlled-release patch, which is composed of a backing layer, a pressure-sensitive adhesive skeleton-type drug-containing reservoir layer and a protective film. The preparation method is as follows:
[0056] (1) Accurately weigh 0.18g granisetron, 0.48g dexamethasone, 0.15g succinic acid, add 6ml ethanol and stir until dissolved, slowly add 1g polyethylene glycol 400 and 0.16g azone and stir until the solution is clear, then add 1.8g Eudragit E100, continue to stir until transparent gel-like medicated pressure-sensitive adhesive reservoir viscose;
[0057] (2) Heat the above-mentioned drug-containing pressure-sensitive adhesive reservoir viscose until the ethanol volatilizes to obtain a drug-containing glue with a suitable consistency, degas it by ultrasonic, and apply it on a certain area of CoTran by salivating process TM Put it on the 9720 polyethylene backing film, put it in an oven at 60°C to cure for 40 mi...
Embodiment 3
[0059] Prepare granisetron-dexamethasone compound transdermal controlled-release patch, which is composed of a backing layer, a pressure-sensitive adhesive skeleton type drug-containing reservoir layer and a protective film. The preparation method is as follows:
[0060] (1) Accurately weigh 0.18g granisetron, 0.48g dexamethasone, 0.16g citric acid, add 6ml ethanol and stir until dissolved, slowly add 1g dibutyl sebacate and 0.16g azone and stir until the solution is clear, then add 1.8g of Eudragit E100, continue to stir until the viscose of the drug-containing pressure-sensitive adhesive reservoir is transparent; Ultrasonic degassing, CoTran coated on a certain area by salivation process TM Put it on the 9720 polyethylene backing film, put it in an oven at 60°C to cure for 40 minutes, and laminate it with inert siliconized release paper after cooling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com