Preparation method of calcium aluminosilicate blue phosphor powder for near-ultraviolet excited LED (Light Emitting Diode)
A blue phosphor, calcium aluminosilicate technology, applied in luminescent materials, chemical instruments and methods, use of gas discharge lamps, etc., can solve the problem of the decline in performance and luminous performance of fluorescent powder, long calcination time, and low luminous efficiency and other problems, to achieve the effects of good chemical stability and thermal stability, good crystallinity and high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
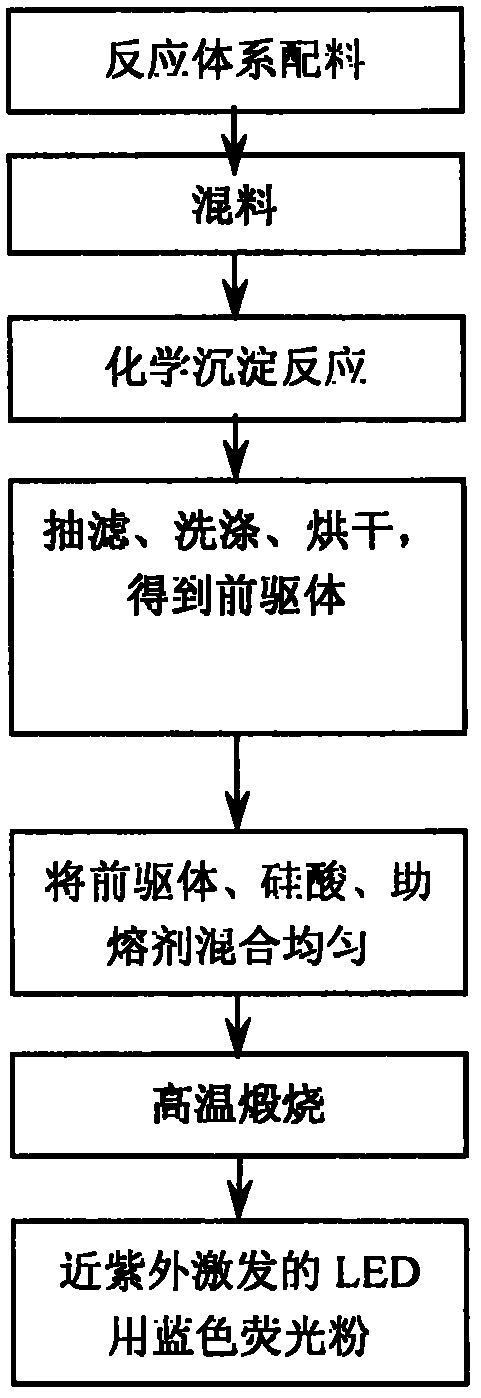
Image
Examples
Embodiment 1
[0023] According to chemical formula Ca 0.985 Al 2 Si 2 O 8 :0.015Eu 2+ Weigh Ca(NO 3 ) 2 (A.R.)0.985mol, Al(NO 3 ) 3 ·9H 2 O(A.R.)2mol, H 2 SiO 3 (A.R.)2mol and Eu(NO 3 ) 3 ·6H 2 O(A.R.) 0.015mol; then weigh 0.1wt% of the surfactant sodium dodecylbenzene sulfonate and flux H of the total mass of the above drugs 3 BO 3 ;Ca(NO 3 ) 2 , Al(NO 3 ) 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. Mix the surfactants evenly and prepare a solution to make it fully dissolved. Weigh NH 4 HCO 3 (1.2 times the theoretical reaction volume), prepare NH 4 HCO 3 Solution, slowly drip Ca(NO 3 ) 2 , Al(NO 3 ) 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. In the surfactant mixture, stir continuously to fully precipitate calcium, aluminum, and europium ions; filter, wash, and dry to obtain the precursor powder; mix the precursor powder, silicic acid, and flux evenly Afterwards, it is calcined in a reducing atmosphere provided by the combustion of activated carbon powder, the calcining temperature is 1300°C...
Embodiment 2
[0025] According to chemical formula Ca 0.975 Al 2 Si 2 O 8 :0.025Eu 2+ Weigh CaCl separately 2 (A.R.) 0.975mol, AlCl 3 ·9H 2 O(A.R.)2mol, H 2 SiO 3 (A.R.)2mol and Eu(NO 3 ) 3 ·6H 2 O(A.R.)0.025mol; then weigh 0.3wt% of the total mass of the above drugs, the surfactant sodium dodecylbenzene sulfonate and the flux MgF 2 ; CaCl to be weighed 2 , AlCl 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. Mix the surfactants evenly and prepare a solution so that it can be fully dissolved. Weigh NH 4 HCO 3 (1.3 times the theoretical reaction volume), prepare NH 4 HCO 3 Solution, slowly drip into CaCl 2 , AlCl 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. In the surfactant mixture, stir constantly to fully precipitate calcium, aluminum and europium ions; filter, wash, and dry to obtain the precursor powder; mix the precursor powder with silicic acid and flux evenly Afterwards, it is calcined in a reducing atmosphere provided by the combustion of activated carbon powder, the calcining temperature is...
Embodiment 3
[0027] According to chemical formula Ca 0.965 Al 2 Si 2 O 8 :0.035Eu 2+ Weigh Ca(NO 3 ) 2 (A.R.)0.965mol, Al(NO 3 ) 3 ·9H 2 O(A.R.)2mol, H 2 SiO 3 (A.R.)2mol and Eu(NO 3 ) 3 ·6H 2 O(A.R.) 0.035mol; then weigh 0.5wt% of the surfactant polyethylene glycol and flux H of the total mass of the above drugs 3 BO 3 ;Ca(NO 3 ) 2 , Al(NO 3 ) 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. Mix the surfactants evenly and prepare a solution so that it can be fully dissolved. Weigh NH 4 HCO 3 (1.4 times the theoretical reaction volume), to prepare NH 4 HCO 3 Solution, slowly drip Ca(NO 3 ) 2 , Al(NO 3 ) 3 ·9H 2 O, H 2 SiO 3 , Eu(NO 3 ) 3 ·6H 2 O. In the surfactant mixture, stir continuously to fully precipitate calcium, aluminum and europium ions; filter, wash, and dry to obtain the precursor powder; mix the precursor powder, silicic acid, and flux evenly Afterwards, it is calcined in a reducing atmosphere provided by the combustion of activated carbon powder at a calcining temperature of 1150°C an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com