Preparation method of ethyecellulose-based macromonomer
An ethyl cellulose and macromonomer technology is applied in the field of preparation of ethyl cellulose-based macromonomers, which can solve the problems of limiting cellulose modification and processing and utilization, and achieve high polymerization activity and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the first step, ethyl cellulose is dissolved in an organic solvent, and the solvent can be selected such as tetrahydrofuran, dimethylformamide, dimethylacetamide, dioxane, dimethyl sulfoxide, and under low temperature conditions, carry out Ultrasonic activation treatment; temperature optional -20°C, -18°C, -16°C, -14°C, -12°C, -14°C, -10°C, -8°C, -6°C, -4°C, -2°C , 0°C, ultrasonic time is 5~60s, optional 5s, 10s, 15s, 20s, 25s, 30s, 35s, 40s, 45s, 50s, 55s, 60s, ultrasonic interval time is 1~10min, ultrasonic interval time is optional 1min, 2min, 3min, 4min, 5min, 50min, 6min, 7min, 8min, 9min, 10min. The duration of the ultrasonic activation process is 10-60min, 10min, 15min, 20min, 25min, 30min, 35min, 40min, 45min, 50min, 55min, 60min are optional.
[0025] In the second step, catalyst and polymerization inhibitor hydroquinone are added for further low-temperature activation and nitrogen deoxygenation treatment. The catalyst can be selected from any one of N, N-...
Embodiment 2
[0028] Synthetic steps:
[0029]
[0030] In the first step, 1.0 g of ethyl cellulose was dissolved in 50 mL of tetrahydrofuran, and ultrasonic activation was performed at 0 ° C. The ultrasonic time was 10 s, the interval time was 2 min, and the ultrasonic duration was 10 min;
[0031] In the second step, add catalyst triethylamine 2.2mL and polymerization inhibitor hydroquinone 0.001g, further low-temperature activation and nitrogen deoxygenation treatment at 0°C for 30min;
[0032] In the third step, at 0°C, add 1.3 mL of acryloyl chloride dropwise (acryloyl chloride / ethyl cellulose hydroxyl molar ratio = 3:1, keep at 0°C for 2 hours, then raise the temperature to room temperature for 15 hours; then the solution drop into water for precipitation, filter, wash with water and freeze-dry to obtain ethyl cellulose-based macromer with a substitution degree of 0.60.
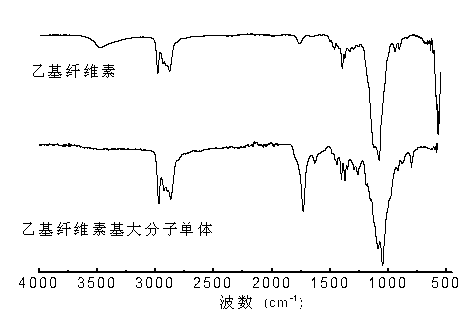
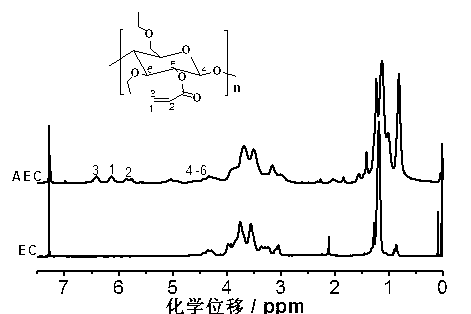
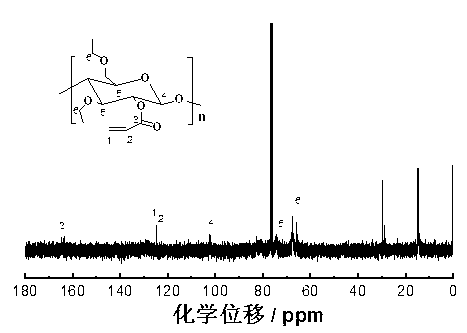
[0033] Using infrared spectroscopy (FTIR) ( figure 1 ), H NMR spectrum ( 1 H NMR) ( figure 2 ) C-13 NMR sp...
Embodiment 3
[0036] In the first step, dissolve 1.0 g of ethyl cellulose in 50 mL of tetrahydrofuran, and perform ultrasonic activation treatment at -10 ° C. The ultrasonic time is 10 s, the interval time is 2 min, and the ultrasonic duration is 10 min;
[0037] In the second step, add catalyst triethylamine 2.2mL and polymerization inhibitor hydroquinone 0.001g, further low-temperature activation and nitrogen deoxygenation treatment at 0°C for 40min;
[0038] The third step is to add 1.3 mL of acryloyl chloride dropwise at 0°C (acryloyl chloride / ethyl cellulose hydroxyl = 3:1, keep the reaction at 0°C for 2 hours, then raise the temperature to room temperature for 15 hours; then drop the solution into Precipitate in water, filter, freeze-dry after washing with water to obtain ethyl cellulose-based macromer. Test result: substitution degree 0.65.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com