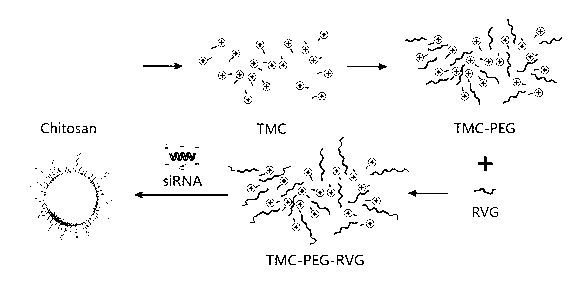
Trimethyl chitosan-graft-polyethylene glycol/nucleic acid brain-targeting micellar and preparation method thereof
A technology of trimethyl chitosan and polyethylene glycol, which can be used in gene therapy, pharmaceutical formulations, non-effective ingredients of polymer compounds, etc., and can solve problems such as easy degradation and unstable transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0045] The purification of example 1 chitosan
[0046] Add 13.8g of chitosan into 1120mL of 1% acetic acid aqueous solution, stir magnetically for 40 minutes to dissolve it, filter it with suction, add ammonia water dropwise to adjust the pH value of the filtrate to 7.0 to precipitate chitosan, and filter it with a 0.45 μm filter membrane. The chitosan product on the filter membrane was washed 3 times with double distilled water, and freeze-dried.
example 2
[0047] The preparation of example 2 N-trimethyl chitosan
[0048] sample 1
[0049] Weigh 0.5 g of purified chitosan (molecular weight 50 kD, degree of deacetylation 95%) into a 50 mL three-necked flask, add 20 mL of N-methylpyrrolidone, and stir and swell at room temperature for 12 hours. Add 1.2 g of sodium iodide to 2.8 mL with a mass concentration of 0.2 g·mL -1 sodium hydroxide solution and 3 mL of methyl iodide, and stirred in a water bath at 60 °C for 45 minutes to complete the first step of methylation. Add 2.8 mL of sodium hydroxide solution and 1.25 mL of methyl iodide to react for 45 minutes to complete the second step of methylation. The reaction solution was added to 200 mL of absolute ethanol and stirred for 1 hour to precipitate trimethyl chitosan. The precipitate was centrifuged (5000 rpm, 15 minutes) and washed three times with ethanol, three times with ether, and then dried in vacuum. After drying, dissolve the product in 20 mL of 50 g L -1 I ions were ex...
example 3 3
[0056] The preparation of example 3 trimethyl chitosan-graft-polyethylene glycol
[0057] sample 1
[0058] 10 mg of trimethyl chitosan and 15 mg of maleimide-polyethylene glycol-succinimide (MAL-PEG-SCM) of sample 1 in Example 2 were dissolved in 2 mL of distilled water, and magnetically stirred at room temperature for 6 hours , ultrafiltration and centrifugation to remove unreacted PEG modifiers, the obtained concentrated solution was diluted with distilled water and then ultrafiltration and centrifugation, repeated 3 times, and finally the ultrafiltrate was freeze-dried.
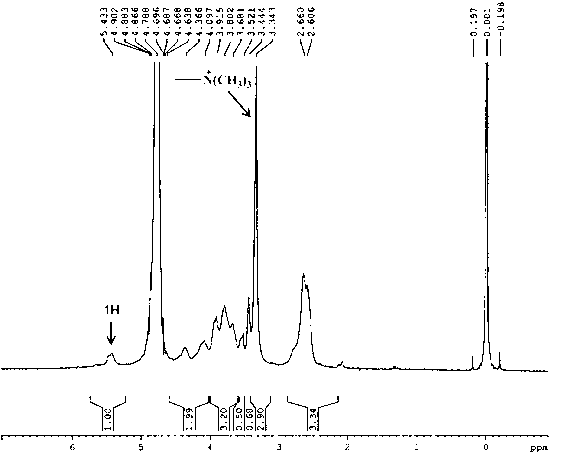
[0059] Get the trimethyl chitosan (TMC) of example 2 sample 1, the synthetic trimethyl chitosan-graft-polyethylene glycol (TMC-PEG-MAL) and maleimide-polyethylene glycol - Succinimide (MAL-PEG-SCM) 5 mg each, in D 2 O is the solvent 1 H-NMR (300 MHz) characterization, the results entered Figure 4 . Figure 4 Compared with TMC in TMC-PEG-MAL, the extra peak with a chemical shift of 3.713 is -CH in po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com