Intestine immunomodulator
An immunomodulatory, intestinal technology, applied in food science, antibacterial drugs, applications, etc., can solve problems such as aggravation of symptoms and insufficient mucosal protection, and achieve the effect of preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Lactobacillus paracasei K71 was inoculated into the MRS medium, and cultured at 37° C. for 20 hours. Lactic acid cells were recovered by centrifugation, washed twice with distilled water, dextrin equal to the weight of dry cells was added, and heat-sterilized at 80° C. for 30 minutes. The dead cell powder of Lactobacillus paracasei K71 (hereinafter abbreviated as "K71 powder") was obtained after freeze-drying.
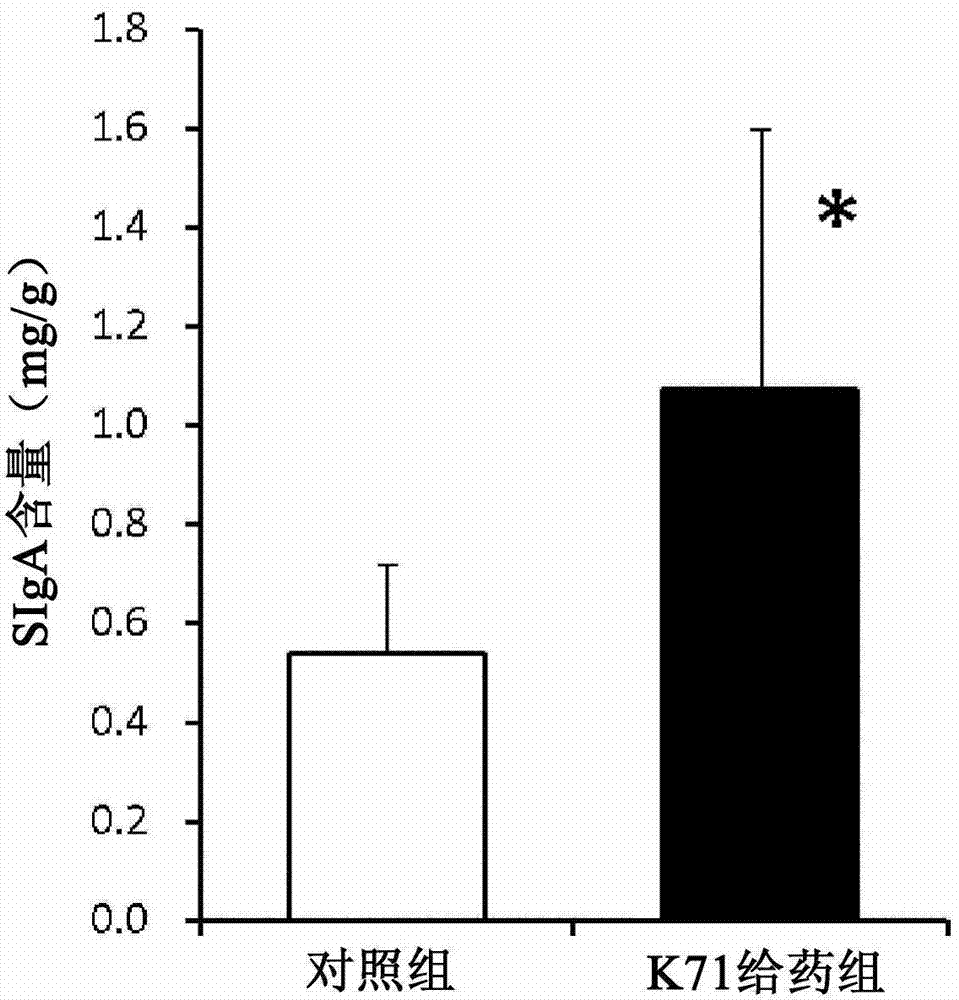
[0048] BALB / c female mice (5 weeks old, purchased from Charles River Company) were acclimatized and raised in a normal environment at 22°C for one week, and then divided into two groups, with 5 mice in each group. Suspend 20 mg of K71 powder in 1 mL of sterilized water, and administer 50 μL of the suspension to one of the groups orally once a day (1 mg-K71 powder / mouse / day) (K71 administration group). Similarly, another group was given 50 μL of sterilized water once a day (control group). In the fifth week of feeding (from the night of the 34th day to the morn...
Embodiment 2
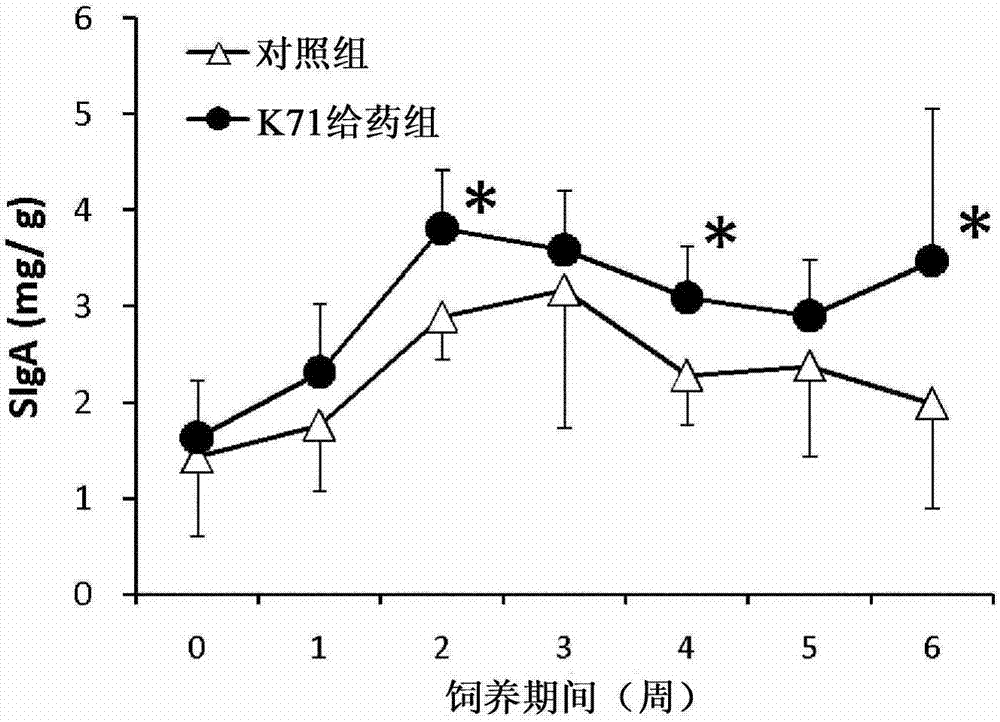
[0051] As in Example 1, 1 mg / mouse / day of K71 powder was repeatedly orally administered to BALB / c female mice (6 mice in each group), and the content of SIgA in feces was measured once a week. As a result, such as figure 2 It is shown that during the whole test period, the SIgA content of the K71 administration group showed a higher value compared with the control group, and the difference was statistically significant in the 2nd, 4th and 6th week (P<0.05).
Embodiment 3
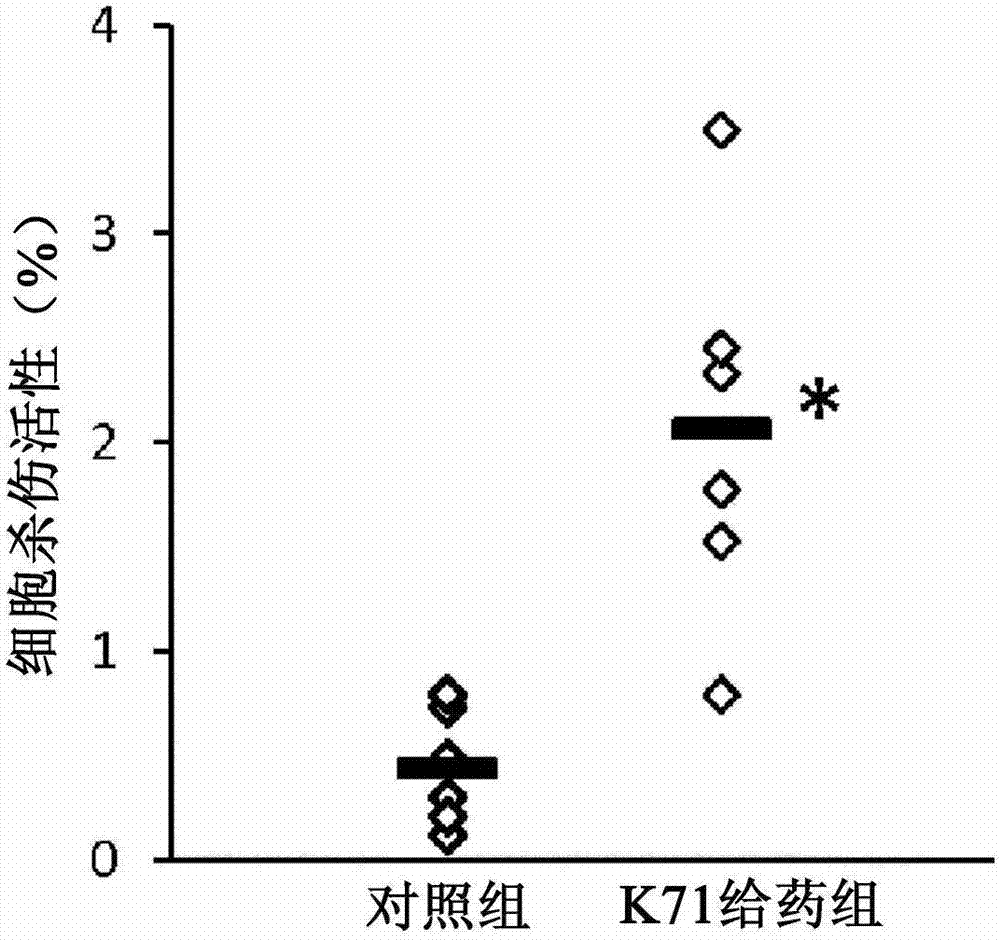
[0053] Same as in Example 1, BALB / c female mice (6 in each group) were given K71 powder or sterilized water every day. Spleen cells were collected after 5 weeks of feeding. Splenocytes (1.25×10 6 cells / ml) and YAC-1 cells (1×10 5 cells / ml) at a ratio of 12.5:1 (spleen cells:YAC-1 cells), in RPMI-1640 medium (96-well culture plate) containing 1% fetal bovine serum, at 37°C, 5% CO 2 conditions for 4 hours. By investigating the activity of lactate dehydrogenase (LDH) released as the YAC-1 cells were destroyed, the cell killing activity of the collected spleen cells was determined. Specifically, 50 μL of the co-culture supernatant of splenocytes and YAC-1 cells was transferred to a new 96-well culture plate, and the LDH enzyme activity was measured using Roche Cytotoxicity Detection Kit (LDH).
[0054] The result is as image 3 As shown, compared with the control group, the cell killing activity of splenocytes in the K71-administered group showed a higher value, which was sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com